
The decreasing order of aromaticity of the following is:
I. Benzene
II. Naphthalene
III. Anthracene
A.(I) > (II) > (III)
B.(III) > (II) > (I)
C.(II) > (I) > (III)
D.(II) > (III) > (I)
Answer
582.6k+ views
Hint: Aromaticity can be understood as the property of conjugated cycloalkanes or alkanes which exhibit the property of resonance, which improves the stability of a molecule due to the delocalization of electrons present in the pi – pi orbitals.
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
For any given molecule to be considered as aromatic, certain criteria must be met by the molecule. These rules can be given as follows:
1.The molecule of the given compound must be cyclic.
2.Each element in the cyclic structure must have a p – orbital that is perpendicular to the ring. This makes the molecule to be planar.
3.The molecule should obey Huckle’s rule
4.The molecular structure of the compound must be flat or planar.
5.The degree of aromaticity depends on the stability of the compound. Higher the stability, higher would be the aromatic character.
The molecular structures of the given compounds are:
Benzene:
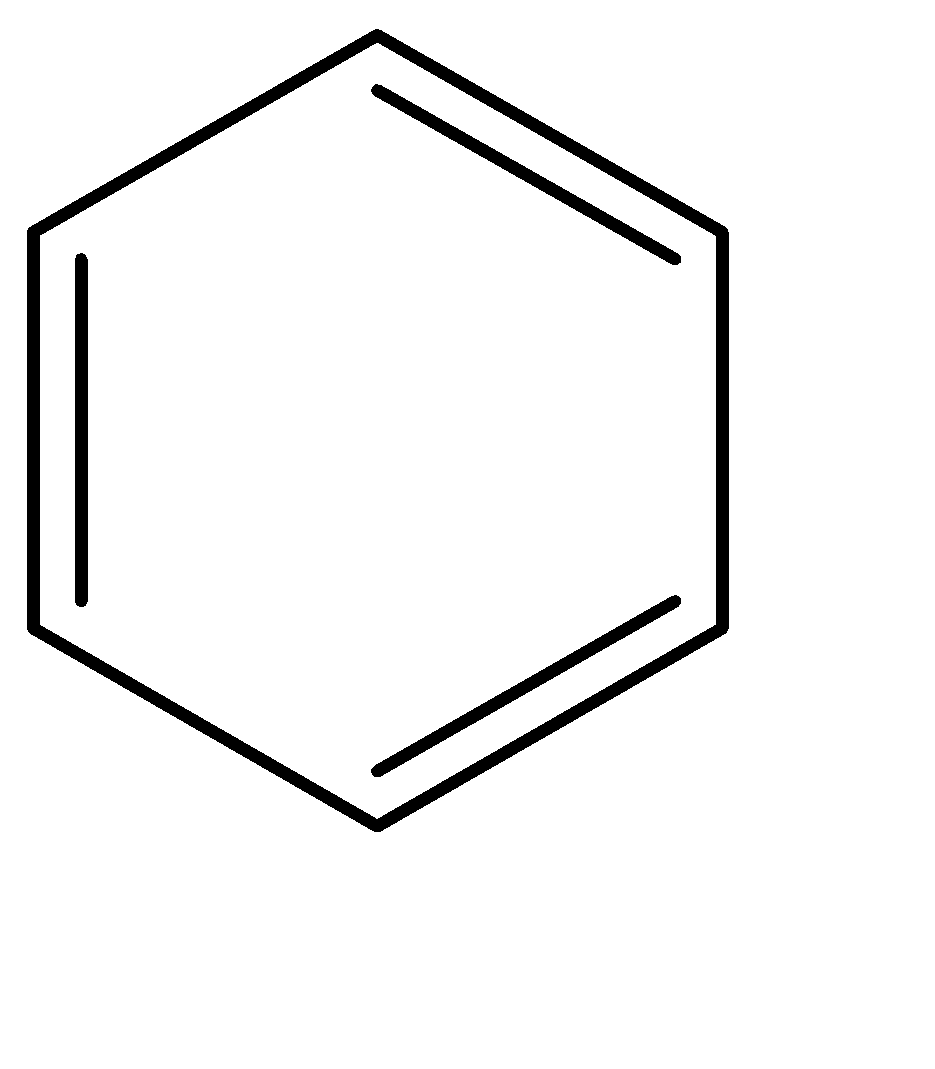
Naphthalene
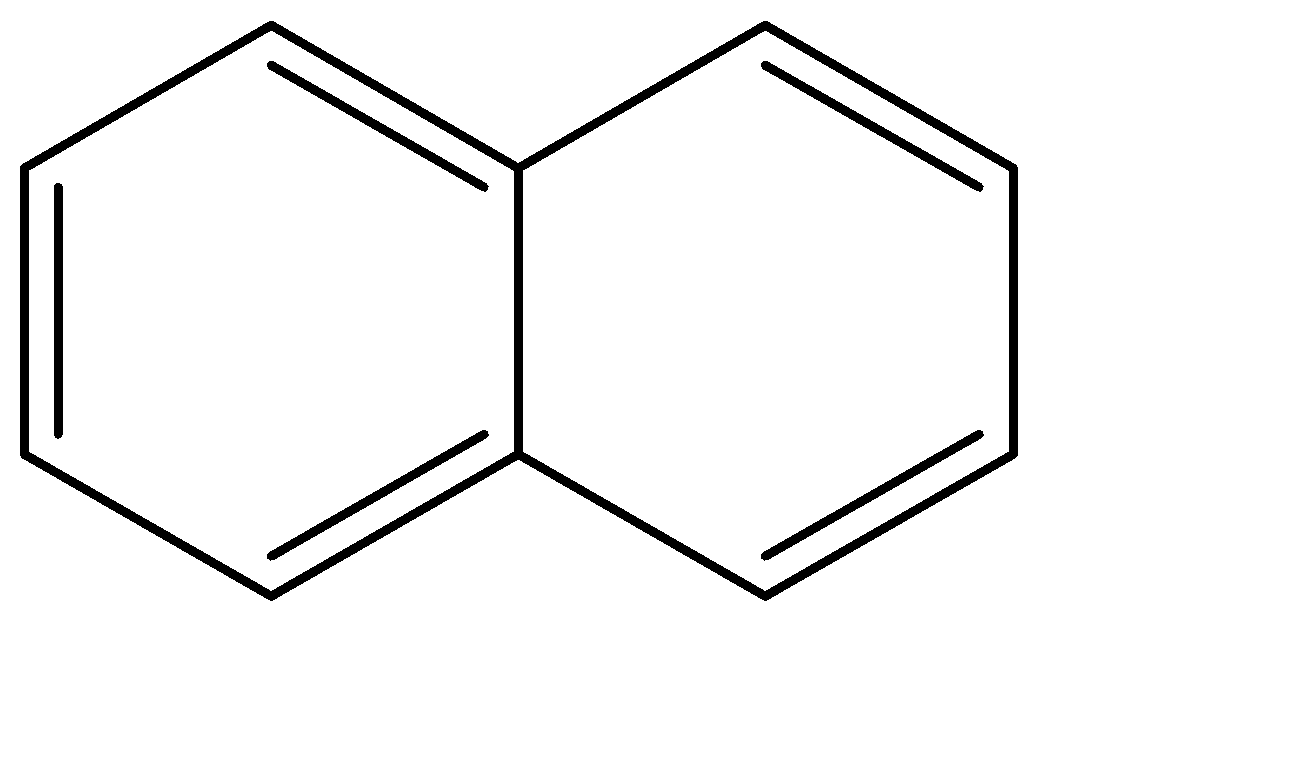
Anthracene:
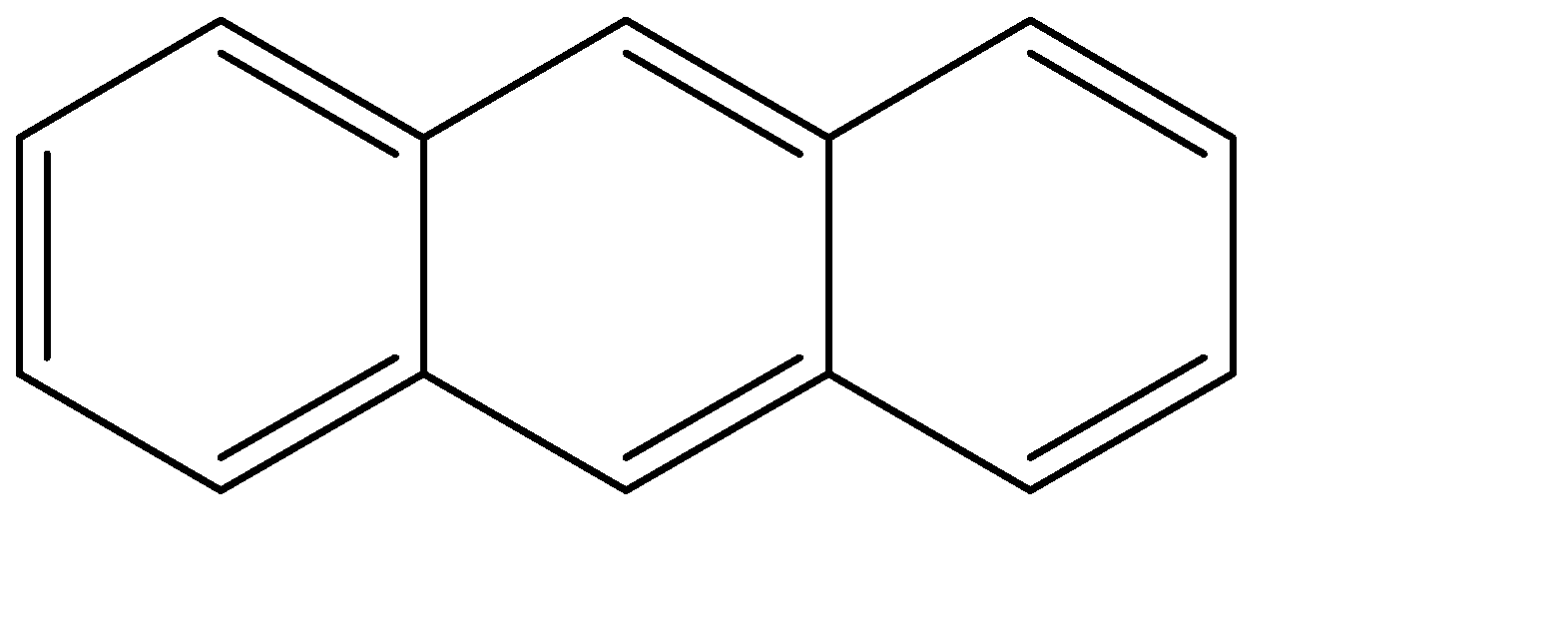
Resonance energy can be understood as the difference between the potential energy of the actual structure and the conjugated structure with the lowest potential. The resonance energy decreases with the increase in the number of rings, and directly contributes to the stability of the rings.
Since the number of rings keeps on increasing from benzene to naphthalene to anthracene, the resonance energy keeps on decreasing. Because of this, the stability as well as the aromaticity also keep on decreasing.
Hence, the decreasing order of aromaticity of the following is (I) > (II) > (III)
Hence, Option A is the correct option.
Note:
From these rules, the one you would probably be unfamiliar with would be Huckel's Rule. Now, Huckle’s rule can be explained as: The molecule must have \[\left( {4n + 2} \right)\] Pi electrons, where ‘n’ is any natural number. Now, another doubt you might have is about pi electrons. Pi electrons are nothing but those electrons which are present in the pi bond of the molecule.
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
For any given molecule to be considered as aromatic, certain criteria must be met by the molecule. These rules can be given as follows:
1.The molecule of the given compound must be cyclic.
2.Each element in the cyclic structure must have a p – orbital that is perpendicular to the ring. This makes the molecule to be planar.
3.The molecule should obey Huckle’s rule
4.The molecular structure of the compound must be flat or planar.
5.The degree of aromaticity depends on the stability of the compound. Higher the stability, higher would be the aromatic character.
The molecular structures of the given compounds are:
Benzene:

Naphthalene

Anthracene:

Resonance energy can be understood as the difference between the potential energy of the actual structure and the conjugated structure with the lowest potential. The resonance energy decreases with the increase in the number of rings, and directly contributes to the stability of the rings.
Since the number of rings keeps on increasing from benzene to naphthalene to anthracene, the resonance energy keeps on decreasing. Because of this, the stability as well as the aromaticity also keep on decreasing.
Hence, the decreasing order of aromaticity of the following is (I) > (II) > (III)
Hence, Option A is the correct option.
Note:
From these rules, the one you would probably be unfamiliar with would be Huckel's Rule. Now, Huckle’s rule can be explained as: The molecule must have \[\left( {4n + 2} \right)\] Pi electrons, where ‘n’ is any natural number. Now, another doubt you might have is about pi electrons. Pi electrons are nothing but those electrons which are present in the pi bond of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




