
The decrease in the pH of the blood it will decreases :
(a) The rate of heartbeat
(b) The blood supply to the brain
(c) The affinity of hemoglobin with oxygen
(d) Release bicarbonate ions by the liver
Answer
537.9k+ views
Hint: It alludes to the perception that will increment inside the carbon dioxide partial pressure of blood or diminishes in blood pH end in a lower affinity. This manifests as a right-ward shift within the gas-Hemoglobin Dissociation Curve delineated in Oxygen Transport.
Complete answer:
A lower pH within the blood will increase dioxide concentration that successively, is implicational an additional active tissue that needs additional gas. In line with the nuclear physicists, the lower pH can cause Hb to deliver additional gas. This manifests as a right-ward shift within the Oxygen-Hemoglobin Dissociation Curve delineated in gas transport and yields increased unloading of gas by Hb.
Decreases in blood pH, which means exaggerated ${H^+}$ concentration, square measure doubtless the direct reason behind lower Hb affinity for gas. Specifically, the association of ${H^+}$ ions with the amino acids of Hb seems to scale back hemoglobin's affinity for gas. As a result of changes within the dioxide partial pressure will modify blood pH, exaggerated partial pressures of carbon dioxide may end in right-ward shifts of the oxygen-hemoglobin dissociation curve. The link between dioxide partial pressure and blood pH is mediated by carbonic anhydrase that converts carbon dioxide to carbonic acid that successively releases a free proton, reducing the native pH of the blood.
So the correct answer is ‘Decreases the affinity of Hb with oxygen’.
Note:
The nuclear physicist impact permits for increased unloading of gas in metabolically active peripheral tissues like physical exercise musculus. exaggerated musculus activity ends up in nativized will increase within the partial pressure of dioxide that successively reduces the local blood pH. attributable to the nuclear physicist impact, this ends up in increased unloading of certain gas by Hb passing through the metabolically active tissue and so improves gas delivery. significantly, the nuclear physicist impact enhances gas delivery proportionately to the metabolic activity of the tissue. As additional metabolism takes place, the dioxide partial pressure will increase so inflicting larger reductions in native pH and successively permitting bigger gas unloading. This can be very true in physical exercise of skeletal muscles which can additionally unharness the carboxylic acid that additionally reduces native blood pH and so enhances the nuclear physicist impact.
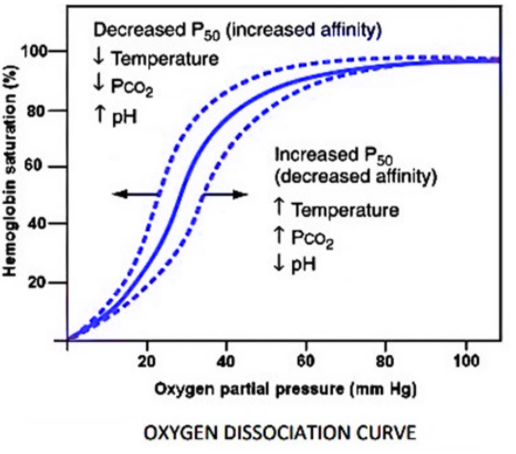
Complete answer:
A lower pH within the blood will increase dioxide concentration that successively, is implicational an additional active tissue that needs additional gas. In line with the nuclear physicists, the lower pH can cause Hb to deliver additional gas. This manifests as a right-ward shift within the Oxygen-Hemoglobin Dissociation Curve delineated in gas transport and yields increased unloading of gas by Hb.
Decreases in blood pH, which means exaggerated ${H^+}$ concentration, square measure doubtless the direct reason behind lower Hb affinity for gas. Specifically, the association of ${H^+}$ ions with the amino acids of Hb seems to scale back hemoglobin's affinity for gas. As a result of changes within the dioxide partial pressure will modify blood pH, exaggerated partial pressures of carbon dioxide may end in right-ward shifts of the oxygen-hemoglobin dissociation curve. The link between dioxide partial pressure and blood pH is mediated by carbonic anhydrase that converts carbon dioxide to carbonic acid that successively releases a free proton, reducing the native pH of the blood.
So the correct answer is ‘Decreases the affinity of Hb with oxygen’.
Note:
The nuclear physicist impact permits for increased unloading of gas in metabolically active peripheral tissues like physical exercise musculus. exaggerated musculus activity ends up in nativized will increase within the partial pressure of dioxide that successively reduces the local blood pH. attributable to the nuclear physicist impact, this ends up in increased unloading of certain gas by Hb passing through the metabolically active tissue and so improves gas delivery. significantly, the nuclear physicist impact enhances gas delivery proportionately to the metabolic activity of the tissue. As additional metabolism takes place, the dioxide partial pressure will increase so inflicting larger reductions in native pH and successively permitting bigger gas unloading. This can be very true in physical exercise of skeletal muscles which can additionally unharness the carboxylic acid that additionally reduces native blood pH and so enhances the nuclear physicist impact.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




