
The correct statement about the synthesis of erythritol $ \left( {C{{\left( {C{H_2}OH} \right)}_4}} \right) $ used in the preparation of PETN is:
(A) The synthesis requires three aldol condensation and one Cannizzaro reaction
(B) Alpha hydrogens of ethanol and methanol are involved in the reaction
(C) The synthesis requires two aldol condensation and two Cannizzaro reaction
(D) The synthesis requires four aldol condensation between methanol and ethanol
Answer
539.7k+ views
Hint :PETN’s full Pentaerythritol tetranitrate. It is also known as PENT, PENTA, TEN or penthrite which is explosive material. PETN is a nitrate ester of pentaerythritol and has structural similarity with nitroglycerin. Generally, Penta refers to the five carbon atoms of the neopentane skeleton. The erythritol is the monomer of PETN.
Complete Step By Step Answer:
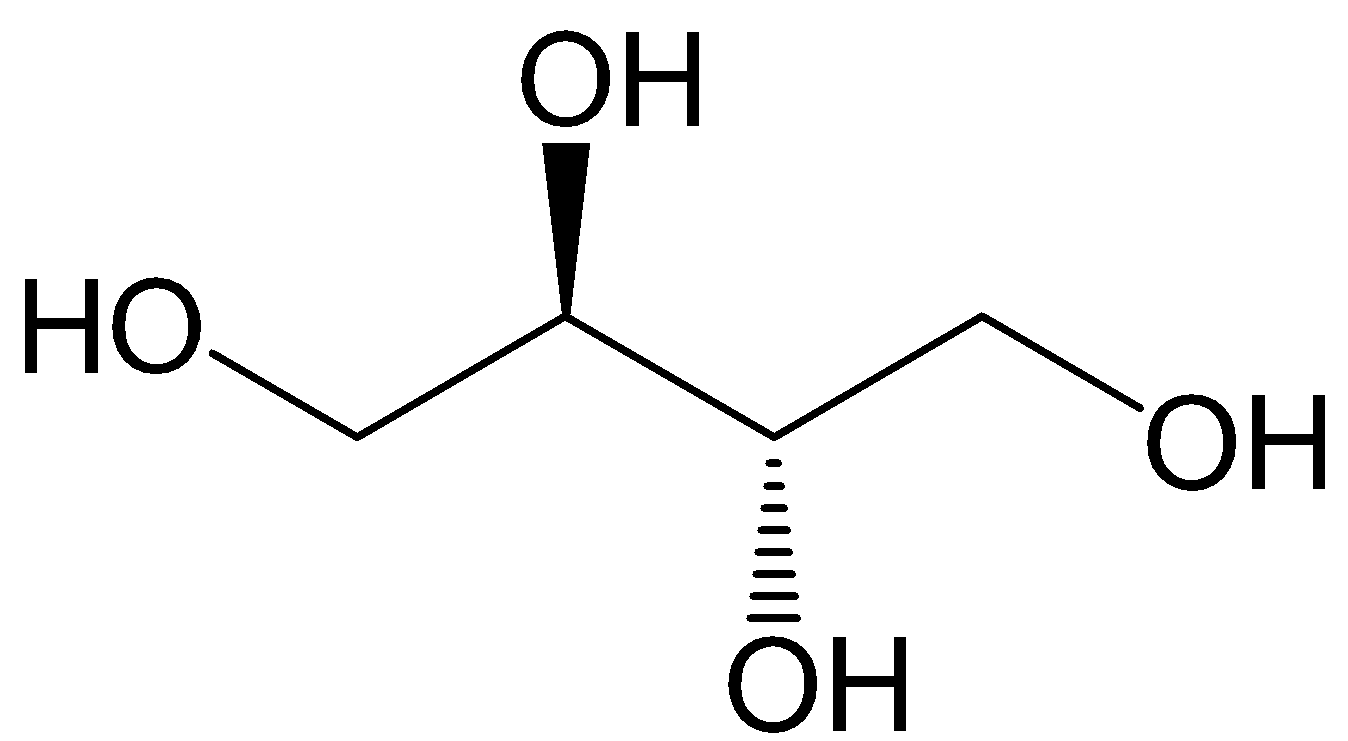
First, let’s observe the structure of erythritol which is having the chemical formula of $ \left( {C{{\left( {C{H_2}OH} \right)}_4}} \right) $ ;
Let’s discuss the aldol condensation;
In aldol condensation, the formation of enolate ion takes place with the carbonyl compound having the $ \alpha - hydrogen $ to form $ \beta - hydroxy\,ketone $ or $ \beta - hydroxy{\text{ }}aldehyde $ , which is followed by dehydration to give a conjugated enone. The aldol condensation plays a major role in organic synthesis, creating a path to form carbon-carbon bonds.
The reaction is given below;
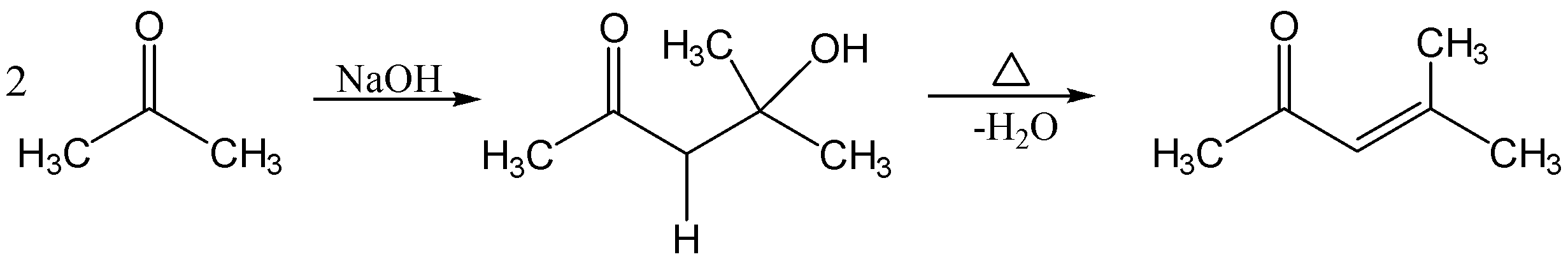
In the reaction, the two moles of acetone react with dilute $ NaOH $ which forms $ \beta - hydroxy ketone $ followed by dehydration to form $ \alpha ,\beta - unsaturated{\text{ }}ketone $ .
Now, let’s see the Cannizzaro reaction;
The Cannizzaro reaction is also known as a disproportionate reaction, in which the two moles of carbonyl compounds not having $ \alpha - hydrogen $ will react with the concentrated base to form alcohol and acid.
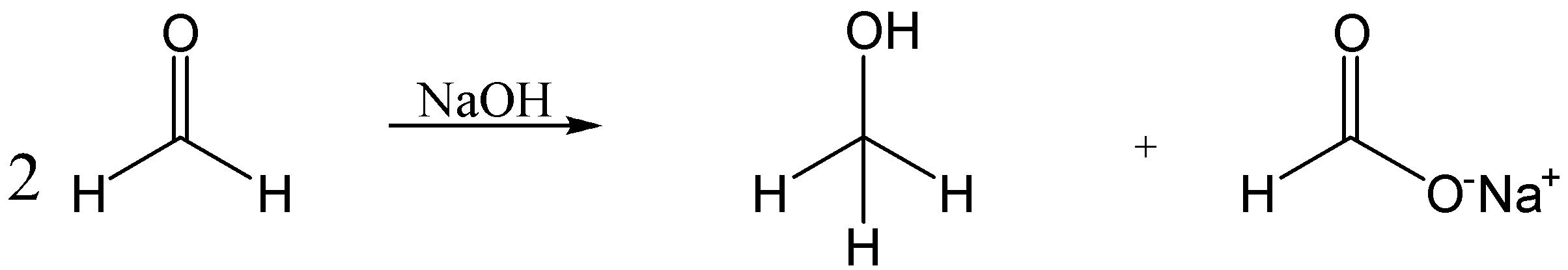
In this reaction, the two moles of formaldehyde react with conc. NaOH to form methanol and sodium formate.
If we observe, we need three times the aldol condensation so that we can obtain the non- $ \alpha - hydrogen $ . Then we need to do Cannizzaro reaction to obtain our desired product erythritol $ \left( {C{{\left( {C{H_2}OH} \right)}_4}} \right) $ ;
The reaction is given below so that we can understand in a better way;
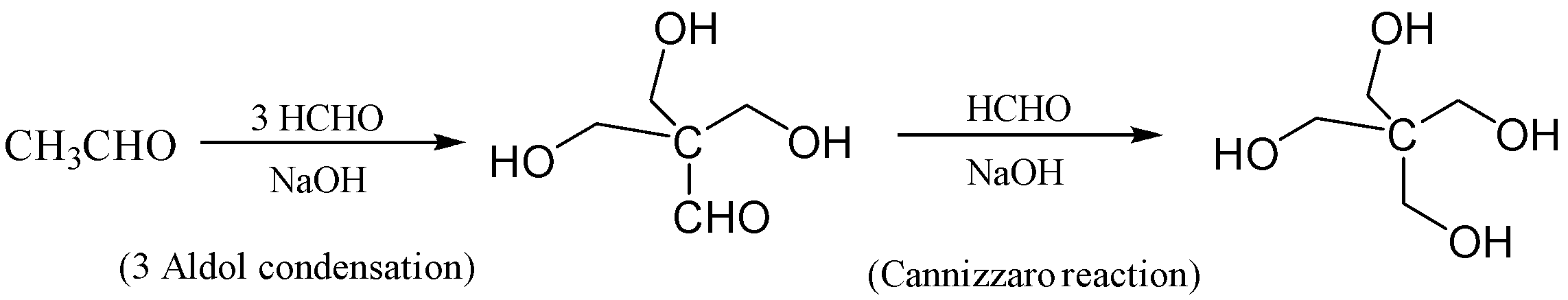
The correct answer is (A) The synthesis requires three aldol condensation and one Cannizzaro reaction
Note :
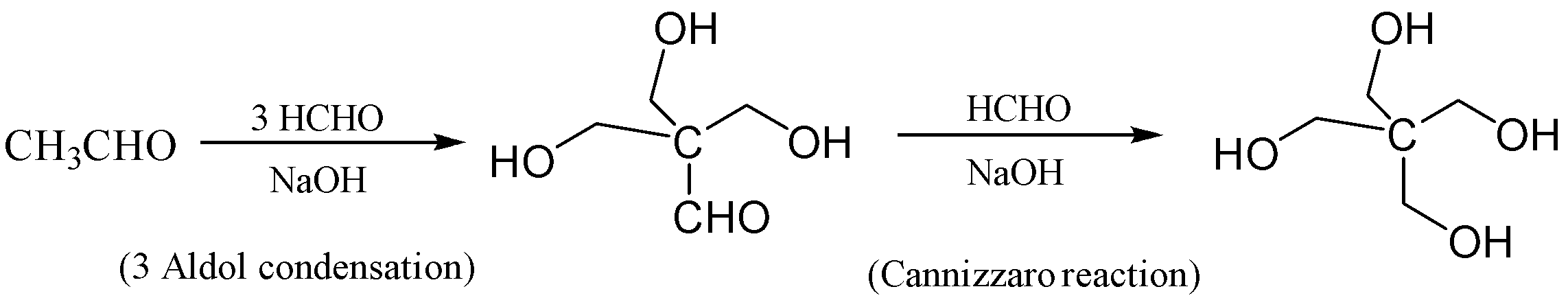
In this reaction, the ethanal is reacted with formaldehyde in the presence of excess sodium hydroxide which forms $ 3 - hydroxy - 2,2 - bis\left( {hydroxymethyl} \right)propanal $ . This reaction occurs thrice which is aldol condensation. In the next step, the Cannizzaro Reaction occurs in which $ 3 - hydroxy - 2,2 - bis\left( {hydroxymethyl} \right)propanal $ is reacted with formaldehyde we get $ 2,2 - bis\left( {hydroxymethyl} \right)propane - 1,3 - diol $ which is also known as erythritol.
Complete Step By Step Answer:
First, let’s observe the structure of erythritol which is having the chemical formula of $ \left( {C{{\left( {C{H_2}OH} \right)}_4}} \right) $ ;

Let’s discuss the aldol condensation;
In aldol condensation, the formation of enolate ion takes place with the carbonyl compound having the $ \alpha - hydrogen $ to form $ \beta - hydroxy\,ketone $ or $ \beta - hydroxy{\text{ }}aldehyde $ , which is followed by dehydration to give a conjugated enone. The aldol condensation plays a major role in organic synthesis, creating a path to form carbon-carbon bonds.
The reaction is given below;

In the reaction, the two moles of acetone react with dilute $ NaOH $ which forms $ \beta - hydroxy ketone $ followed by dehydration to form $ \alpha ,\beta - unsaturated{\text{ }}ketone $ .
Now, let’s see the Cannizzaro reaction;
The Cannizzaro reaction is also known as a disproportionate reaction, in which the two moles of carbonyl compounds not having $ \alpha - hydrogen $ will react with the concentrated base to form alcohol and acid.

In this reaction, the two moles of formaldehyde react with conc. NaOH to form methanol and sodium formate.
If we observe, we need three times the aldol condensation so that we can obtain the non- $ \alpha - hydrogen $ . Then we need to do Cannizzaro reaction to obtain our desired product erythritol $ \left( {C{{\left( {C{H_2}OH} \right)}_4}} \right) $ ;
The reaction is given below so that we can understand in a better way;

The correct answer is (A) The synthesis requires three aldol condensation and one Cannizzaro reaction
Note :

In this reaction, the ethanal is reacted with formaldehyde in the presence of excess sodium hydroxide which forms $ 3 - hydroxy - 2,2 - bis\left( {hydroxymethyl} \right)propanal $ . This reaction occurs thrice which is aldol condensation. In the next step, the Cannizzaro Reaction occurs in which $ 3 - hydroxy - 2,2 - bis\left( {hydroxymethyl} \right)propanal $ is reacted with formaldehyde we get $ 2,2 - bis\left( {hydroxymethyl} \right)propane - 1,3 - diol $ which is also known as erythritol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




