
The correct set of reagents for the following conversion is:
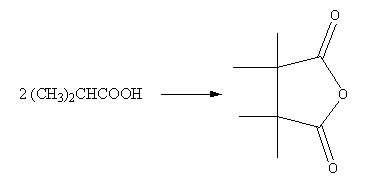
A. ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta ,{P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na}}$
B. ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na, }}{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $
C. ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}{\text{,}}\,{\text{NaB}}{{\text{H}}_{\text{4}}}$
D. ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na,}}\,{\text{conc}}{\text{.}}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$

Answer
578.4k+ views
Hint: We can synthesize the product by ionizing the reactant in the first step then we will condense the iodized reactant and the condensed product will be treated with the reagent which can cause anhydride formation.
Complete step by step answer:
The reaction of ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{2}}}{\text{CHCOOH}}$ with ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ lead to the formation of anhydride but not cyclic anhydride then treating with ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na}}$ leads to a different product so, option (A) is incorrect.
${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}$ is an oxidizing reagent. This reagent gives a nucleophilic substitution reaction. The nucleophile iodide substitutes the hydrogen.
The reaction is shown as follows:
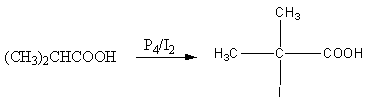
The two iodized reactants can be condensed by using sodium in presence of dry ether.
The reaction is as follows:
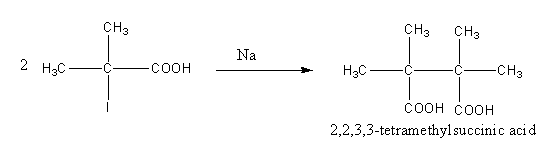
The condensed product is tetramethyl succinic acid.
When two carboxylic groups are condensed they form anhydride. The ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ reagent causes the anhydride formation.
The reaction is as follows:

So, the reactant can be converted into the product by using the ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na, }}{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $.
The reaction of ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{2}}}{\text{CHCOOH}}$ with ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ lead to the formation of anhydride but not cyclic anhydride then treating with ${\text{NaB}}{{\text{H}}_{\text{4}}}$ will give alcohol as a product so, option (C) is incorrect.
The reaction of ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{2}}}{\text{CHCOOH}}$ with ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na}}$ lead to the formation of tetramethyl succinic acid then treating with ${\text{conc}}{\text{.}}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ leads to a different product so, option (D) is incorrect.
Therefore, option (B) ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na, }}{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ is correct.
Note: The reagent ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}$ introduced iodine into the reactant. ${\text{conc}}{\text{.}}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ is an oxidizing and dehydrating reagent. ${\text{NaB}}{{\text{H}}_{\text{4}}}$ is a reducing agent. ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ cause anhydride formation. Sodium in dry ether case the joining of aryl halide and alkyl halide or two aryl or two alkyl halides. The reaction is known as Wurtz fittig reaction.
Complete step by step answer:
The reaction of ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{2}}}{\text{CHCOOH}}$ with ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ lead to the formation of anhydride but not cyclic anhydride then treating with ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na}}$ leads to a different product so, option (A) is incorrect.
${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}$ is an oxidizing reagent. This reagent gives a nucleophilic substitution reaction. The nucleophile iodide substitutes the hydrogen.
The reaction is shown as follows:
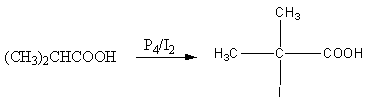
The two iodized reactants can be condensed by using sodium in presence of dry ether.
The reaction is as follows:

The condensed product is tetramethyl succinic acid.
When two carboxylic groups are condensed they form anhydride. The ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ reagent causes the anhydride formation.
The reaction is as follows:

So, the reactant can be converted into the product by using the ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na, }}{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $.
The reaction of ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{2}}}{\text{CHCOOH}}$ with ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ lead to the formation of anhydride but not cyclic anhydride then treating with ${\text{NaB}}{{\text{H}}_{\text{4}}}$ will give alcohol as a product so, option (C) is incorrect.
The reaction of ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{2}}}{\text{CHCOOH}}$ with ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na}}$ lead to the formation of tetramethyl succinic acid then treating with ${\text{conc}}{\text{.}}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ leads to a different product so, option (D) is incorrect.
Therefore, option (B) ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}{\text{,Na, }}{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ is correct.
Note: The reagent ${P_{\text{4}}}{\text{/}}\,{{\text{I}}_{\text{2}}}$ introduced iodine into the reactant. ${\text{conc}}{\text{.}}\,{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ is an oxidizing and dehydrating reagent. ${\text{NaB}}{{\text{H}}_{\text{4}}}$ is a reducing agent. ${{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}/\Delta $ cause anhydride formation. Sodium in dry ether case the joining of aryl halide and alkyl halide or two aryl or two alkyl halides. The reaction is known as Wurtz fittig reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




