
The correct sequence of steps involved in the mechanism of Cannizzaro reaction is:
A. nucleophilic attack, transfer of ${{H}^{-}}$ and transfer of ${{H}^{+}}$
B. transfer of ${{H}^{-}}$, transfer of ${{H}^{+}}$ and nucleophilic attack
C. transfer of ${{H}^{+}}$ nucleophilic attack and transfer of ${{H}^{-}}$
D. electrophilic attack by $O{{H}^{-}}$ transfer of${{H}^{-}}$ and transfer of ${{H}^{-}}$
Answer
585.9k+ views
Hint: Cannizzaro reaction is a reaction in which disproportionation of two molecules of aldehyde takes place to give a primary alcohol and a carboxylic acid, it involves the nucleophilic substitution on aldehydes.
Complete step by step answer:
- We can see from the following mechanism that how Cannizzaro reaction takes place:
- We can see that in step 1, we have, one HCHO and one $O{{H}^{-}}$, which will react together and this will be the fast step of the reaction, we can say that there is nucleophilic attack of $O{{H}^{-}}$ to the carbonyl carbon and we get:
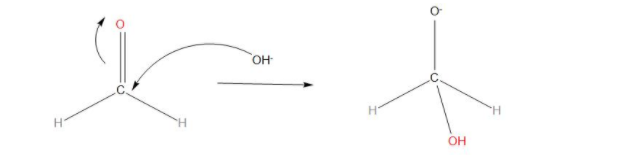
- In step 2, we can see that the transfer of hydride ions from anion to the second molecule of aldehyde takes place. And finally the rapid transfer of protons takes place.
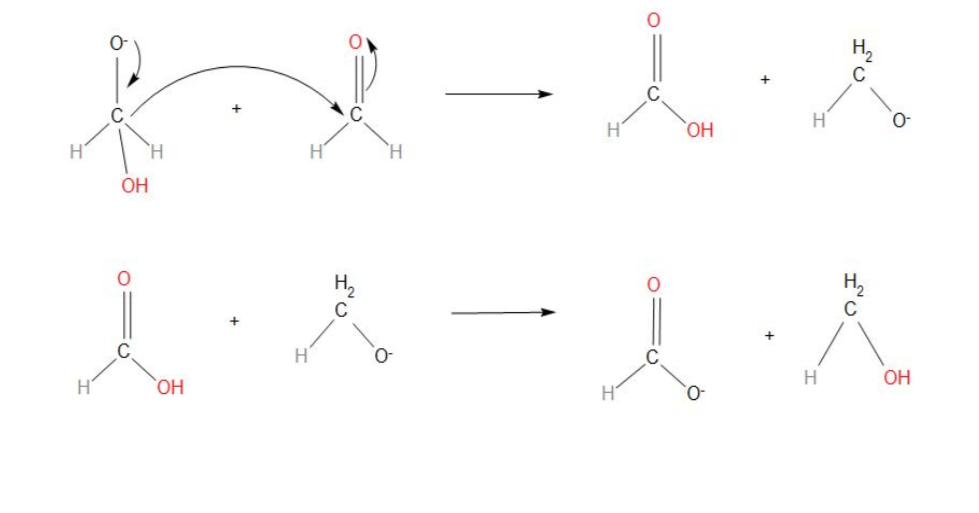
- Hence, we can conclude that the correct sequence of steps involved in the mechanism of Cannizzaro reaction is: nucleophilic attack, transfer of ${{H}^{-}}$ and transfer of ${{H}^{+}}$
So, the correct answer is “Option C”.
Note: - This reaction is always given by aldehydes that doesn’t have an alpha hydrogen atom (it is present on the alpha carbon). For example, acetaldehyde doesn’t undergo a cannizzaro reaction because it has alpha hydrogen.
Complete step by step answer:
- We can see from the following mechanism that how Cannizzaro reaction takes place:
- We can see that in step 1, we have, one HCHO and one $O{{H}^{-}}$, which will react together and this will be the fast step of the reaction, we can say that there is nucleophilic attack of $O{{H}^{-}}$ to the carbonyl carbon and we get:

- In step 2, we can see that the transfer of hydride ions from anion to the second molecule of aldehyde takes place. And finally the rapid transfer of protons takes place.

- Hence, we can conclude that the correct sequence of steps involved in the mechanism of Cannizzaro reaction is: nucleophilic attack, transfer of ${{H}^{-}}$ and transfer of ${{H}^{+}}$
So, the correct answer is “Option C”.
Note: - This reaction is always given by aldehydes that doesn’t have an alpha hydrogen atom (it is present on the alpha carbon). For example, acetaldehyde doesn’t undergo a cannizzaro reaction because it has alpha hydrogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




