
The correct order of strength of the carboxylic acids is
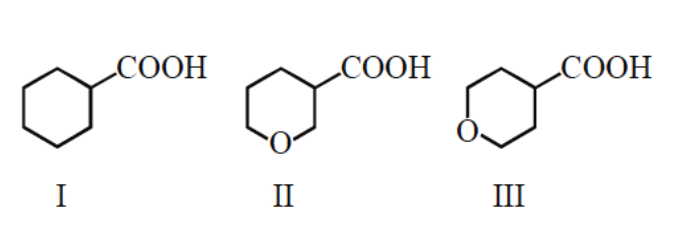
(A) $III > II > I$
(B) $II > I > III$
(C) $I > II > III$
(D) $II > III > I$

Answer
510.3k+ views
Hint:Carboxylic acid is acidic in nature. It is a weak acid. Strength of acid is the tendency of a substance to release ${H^ + }.$If dissociation of acid is completed in an aqueous solution, then it is a strong acid. But carboxylic acid does not dissociate completely in an aqueous solution.
Complete answer:
Carboxylic Acid is a weak acid. But groups attached to –COOH differ in the strength of acid R-COOH.
If the number of C-atoms in the alkyl group increases, the acidity of carboxylic acid decreases.
$HCOOH < C{H_3}COOH < C{H_3}C{H_2}COOH < C{H_3}{(C{H_2})_2}COOH$
If an electron withdrawing substance is present on a C-atom attached to\[-COOH\], acidity increases.
More electronegative atom increases the acidity of carboxylic acid.
Acidity increases as the electronegativity of C-atom, directly attached to –COOH group increases or hybridization change from $s{p^3} \to s{p^2} \to sp$
$HC \equiv C - COOH > C{H_2} = CHCOOH > C{H_3}COOH$
In the given example –COOH is attached to $s{p^3}$ C-atom and $s{p^3}$ hybridized carbon attached to electronegative atoms (oxygen).
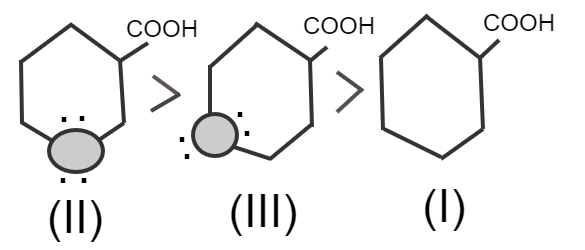
Electronegative atoms of oxygen increase polarity on the –COOH group, so it releases ${H^ + }$easily.
In structure (III) oxygen is away from –COOH so it produces less polarity on the –COOH group, which makes carboxylic acid weaker.
In structure (I) there is no electronegative atom so this is the weakest acid.
Therefore, from the above explanation, the correct option is (D) $II > III > I.$
Note:Carboxylic acid is a weak acid but the presence of substituents affects acidity. Electron withdrawing groups increase acidity and electron releasing groups decrease the acidity of carboxylic acid.
Complete answer:
Carboxylic Acid is a weak acid. But groups attached to –COOH differ in the strength of acid R-COOH.
If the number of C-atoms in the alkyl group increases, the acidity of carboxylic acid decreases.
$HCOOH < C{H_3}COOH < C{H_3}C{H_2}COOH < C{H_3}{(C{H_2})_2}COOH$
If an electron withdrawing substance is present on a C-atom attached to\[-COOH\], acidity increases.
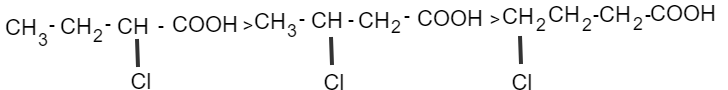
More electronegative atom increases the acidity of carboxylic acid.

Acidity increases as the electronegativity of C-atom, directly attached to –COOH group increases or hybridization change from $s{p^3} \to s{p^2} \to sp$
$HC \equiv C - COOH > C{H_2} = CHCOOH > C{H_3}COOH$
In the given example –COOH is attached to $s{p^3}$ C-atom and $s{p^3}$ hybridized carbon attached to electronegative atoms (oxygen).

Electronegative atoms of oxygen increase polarity on the –COOH group, so it releases ${H^ + }$easily.
In structure (III) oxygen is away from –COOH so it produces less polarity on the –COOH group, which makes carboxylic acid weaker.
In structure (I) there is no electronegative atom so this is the weakest acid.
Therefore, from the above explanation, the correct option is (D) $II > III > I.$
Note:Carboxylic acid is a weak acid but the presence of substituents affects acidity. Electron withdrawing groups increase acidity and electron releasing groups decrease the acidity of carboxylic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




