
The correct order of decreasing acidity of nitrophenols will be:
a. m-nitrophenol
 p-nitrophenol
p-nitrophenol
 o-nitrophenol
o-nitrophenol
b. o-nitrophenol
 m-nitrophenol
m-nitrophenol
 p-nitrophenol
p-nitrophenol
c. p-nitrophenol
 m-nitrophenol
m-nitrophenol
 o-nitrophenol
o-nitrophenol
d. p-nitrophenol
 o-nitrophenol
o-nitrophenol
 m-nitrophenol
m-nitrophenol








Answer
529.3k+ views
Hint: The general formula of nitrophenols is: ${ HOC }_{ 6 }{ H }_{ 5-x }{ (NO }_{ 2 }{ ) }_{ x }$
The structure of nitrophenol is shown below:
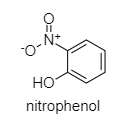
Here the acidity depends on the presence of electron-withdrawing or electron releasing groups and their respective positions.
Complete step-by-step answer:
An inductive effect is an electronic effect due to the polarisation of σ bonds within a molecule or ion. This is typically due to an electronegativity difference between the atoms at either end of the bond.
${ -NO }_{ 2 }$ is an electron-withdrawing group.
${ -NO }_{ 2 }$ group at ortho and para position pulls back electrons of the OH bond towards itself by the stronger -R effect while the ${ -NO }_{ 2 }$ group at m-position pulls back electrons of the OH bond by the weaker effect.
Thus, ortho and para-nitrophenols are more acidic than m-nitrophenol is a little less acidic than p-nitrophenol because of intramolecular hydrogen bonding which makes the loss of a proton difficult to remove.
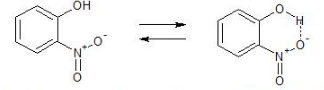
Figure: Showing intramolecular hydrogen bonding in m-nitrophenol.
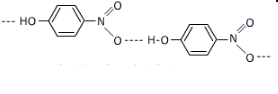
Figure: Showing intermolecular hydrogen bonding in p-nitrophenol
Hence, The correct order of decreasing acidity of nitrophenols will be:
p-nitrophenol
 o-nitrophenol
o-nitrophenol
 m-nitrophenol
m-nitrophenol
The correct option is D.
Additional Information:
Resonance effect: It can be defined as the delocalization of π electrons due to the presence of electron releasing or electron-withdrawing substituents, which results in canonical forms with different electron distribution.
It is designated as the R or M effect.
Application: It gives extra stability to the compound by delocalization of charges. Some time it activates or deactivates the benzene ring towards electrophilic substitution.
Note: The possibility to make a mistake is that you may choose option C. But p-nitrophenol shows both -R and -I effects, so it will be more acidic than m-nitrophenol as it does not show any such effect.
The structure of nitrophenol is shown below:

Here the acidity depends on the presence of electron-withdrawing or electron releasing groups and their respective positions.
Complete step-by-step answer:
An inductive effect is an electronic effect due to the polarisation of σ bonds within a molecule or ion. This is typically due to an electronegativity difference between the atoms at either end of the bond.
${ -NO }_{ 2 }$ is an electron-withdrawing group.
${ -NO }_{ 2 }$ group at ortho and para position pulls back electrons of the OH bond towards itself by the stronger -R effect while the ${ -NO }_{ 2 }$ group at m-position pulls back electrons of the OH bond by the weaker effect.
Thus, ortho and para-nitrophenols are more acidic than m-nitrophenol is a little less acidic than p-nitrophenol because of intramolecular hydrogen bonding which makes the loss of a proton difficult to remove.

Figure: Showing intramolecular hydrogen bonding in m-nitrophenol.

Figure: Showing intermolecular hydrogen bonding in p-nitrophenol
Hence, The correct order of decreasing acidity of nitrophenols will be:
p-nitrophenol


The correct option is D.
Additional Information:
Resonance effect: It can be defined as the delocalization of π electrons due to the presence of electron releasing or electron-withdrawing substituents, which results in canonical forms with different electron distribution.
It is designated as the R or M effect.
Application: It gives extra stability to the compound by delocalization of charges. Some time it activates or deactivates the benzene ring towards electrophilic substitution.
Note: The possibility to make a mistake is that you may choose option C. But p-nitrophenol shows both -R and -I effects, so it will be more acidic than m-nitrophenol as it does not show any such effect.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




