
The correct name of the following polymer is :
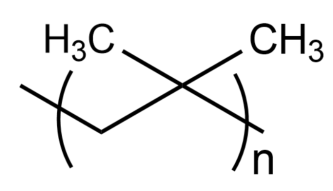
(A)- Polyisoprene
(B)- Polyisobutene
(C)- Polytert-butylene
(D)- Polyisobutylene

Answer
578.1k+ views
Hint:. The given structure shows a polymer molecule whose monomer has three methyl groups attached to a carbon atom. So, we will answer this question by considering the structure of monomers of the compounds given to us as options.
Complete step by step answer:
-Polyisoprene is a polymer molecule produced by the polymerization of isoprene (also known as 2-methyl-1,3-butadiene). The chemical formula of isoprene is $C{{H}_{2}}=C(C{{H}_{3}})-CH=C{{H}_{2}}$, so the given structure cannot be polyisoprene.
-Polyisobutene is a polymer molecule produced by the polymerization of isobutene (also known as 2-methylpropene). The chemical formula of isobutene is ${{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}$, so the given structure cannot be polyisobutene.
-Polytert-butylene have another name as Poly[1-(1,1-dimethyl ethyl)ethylene] which is produced from the polymerization of tert-butyl ethylene whose chemical formula is ${{(C{{H}_{3}})}_{3}}C-CH=C{{H}_{2}}$, so the given structure cannot be polytert-butylene.
-Polyisobutylene is a polymer molecule which is produced by the polymerization of isobutene (also known as 2-methylpropene). The chemical formula of isobutene is ${{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}$, which is the same as the molecule given in the question.
So, the correct answer is “Option D”.
Additional Information:
-Polyisobutylene is a viscous liquid having a molecular weight between 10,000–50,000 with rubbery and amorphous products having a molecular weight of 70,000–225,000 that have a proper cold flow. Its softening point is $185{}^\circ C-200{}^\circ C$and they do not further decompose to $350{}^\circ C$, although its mechanical properties degrade significantly even at $100{}^\circ C$; they retain their elasticity down to $-50{}^\circ C$.
-They are sometimes known as butyl rubber, and also PIB is a vinyl polymer. It's very common to polyethene and polypropylene in structure-wise, apart from that every other carbon is substituted with the other two methyl groups and it is made from the monomer isobutylene, by the process of cationic vinyl polymerization
-Polyisobutylene was first manufactured during the early 1940s. At that time, the most commonly used rubber was natural rubber, polyisoprene. Polyisoprene was also an excellent elastomer, and very likely to isolate from the sap of the hevea trees. Large scale plantations began in Malaysia and grew hevea trees to supply to the world's rubber needs.
-In the industries, polyisobutylene is manufactured by the process ionic polymerization of the monomer at temperatures between $-80{}^\circ \text{ to }-100{}^\circ C$ approximately; they are further processed using the ordinary equipment of the rubber industry. They can thus combine easily with natural and also with the synthetic rubbers such as polyethylene, polyvinyl chloride, and phenol-formaldehyde resins.
-It is a versatile, non-toxic, water-white viscous liquid and can increase the tackiness of it, to provide water-repellency property of it, also to improve viscosity-index of it and it provides excellent grade electrical insulation.
Note: Let us now see some of the common applications of polyisobutylene.
(i) Lubricants: 2-stroke Engine oils, Gear oils, Greases, Hydraulic and Metalworking Fluids.
(ii) Adhesives: It is used as Pressure Sensitive (PSA) or Hot Melt Adhesives (HMA).
(iii) Caulks & Sealants: In the areas of Automotive, Window and Tire Sealant.
(iv) Stretch Wrap: Application including the Tear Resistance improvement, Food Contact.
(v) Electrical Insulation: For the hydrophobic insulation with no electrical conductivity or no oxidation.
Complete step by step answer:
-Polyisoprene is a polymer molecule produced by the polymerization of isoprene (also known as 2-methyl-1,3-butadiene). The chemical formula of isoprene is $C{{H}_{2}}=C(C{{H}_{3}})-CH=C{{H}_{2}}$, so the given structure cannot be polyisoprene.
-Polyisobutene is a polymer molecule produced by the polymerization of isobutene (also known as 2-methylpropene). The chemical formula of isobutene is ${{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}$, so the given structure cannot be polyisobutene.
-Polytert-butylene have another name as Poly[1-(1,1-dimethyl ethyl)ethylene] which is produced from the polymerization of tert-butyl ethylene whose chemical formula is ${{(C{{H}_{3}})}_{3}}C-CH=C{{H}_{2}}$, so the given structure cannot be polytert-butylene.
-Polyisobutylene is a polymer molecule which is produced by the polymerization of isobutene (also known as 2-methylpropene). The chemical formula of isobutene is ${{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}$, which is the same as the molecule given in the question.
So, the correct answer is “Option D”.
Additional Information:
-Polyisobutylene is a viscous liquid having a molecular weight between 10,000–50,000 with rubbery and amorphous products having a molecular weight of 70,000–225,000 that have a proper cold flow. Its softening point is $185{}^\circ C-200{}^\circ C$and they do not further decompose to $350{}^\circ C$, although its mechanical properties degrade significantly even at $100{}^\circ C$; they retain their elasticity down to $-50{}^\circ C$.
-They are sometimes known as butyl rubber, and also PIB is a vinyl polymer. It's very common to polyethene and polypropylene in structure-wise, apart from that every other carbon is substituted with the other two methyl groups and it is made from the monomer isobutylene, by the process of cationic vinyl polymerization
-Polyisobutylene was first manufactured during the early 1940s. At that time, the most commonly used rubber was natural rubber, polyisoprene. Polyisoprene was also an excellent elastomer, and very likely to isolate from the sap of the hevea trees. Large scale plantations began in Malaysia and grew hevea trees to supply to the world's rubber needs.
-In the industries, polyisobutylene is manufactured by the process ionic polymerization of the monomer at temperatures between $-80{}^\circ \text{ to }-100{}^\circ C$ approximately; they are further processed using the ordinary equipment of the rubber industry. They can thus combine easily with natural and also with the synthetic rubbers such as polyethylene, polyvinyl chloride, and phenol-formaldehyde resins.
-It is a versatile, non-toxic, water-white viscous liquid and can increase the tackiness of it, to provide water-repellency property of it, also to improve viscosity-index of it and it provides excellent grade electrical insulation.
Note: Let us now see some of the common applications of polyisobutylene.
(i) Lubricants: 2-stroke Engine oils, Gear oils, Greases, Hydraulic and Metalworking Fluids.
(ii) Adhesives: It is used as Pressure Sensitive (PSA) or Hot Melt Adhesives (HMA).
(iii) Caulks & Sealants: In the areas of Automotive, Window and Tire Sealant.
(iv) Stretch Wrap: Application including the Tear Resistance improvement, Food Contact.
(v) Electrical Insulation: For the hydrophobic insulation with no electrical conductivity or no oxidation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




