
The correct IUPAC name of \[M{n_3}{(CO)_{12}}\] is:
A.ManganeseDodecylCarbonyl (0)
B.Dodecacarbonylmanganate (0)
C.Dodecacarbonylmanganic (II)
D.Dodecacarbonyltrimanganese (0)
Answer
581.1k+ views
Hint: One can solve this question by keeping in mind the IUPAC nomenclature rules. Coordination compounds are neutral and have at least one ion in the complex. They consist of a central metal atom and ligands. To name these compounds, ligands are named first, followed by the metal atom or ion.
Complete step by step answer:
A complex is a substance in which a metal atom or ion in the centre is associated with a group of ligands. Ligands may be neutral or anionic. The coordination compounds are named as per IUPAC nomenclature rules.
In naming the complex ion, the name of the ligand is written first in alphabetical order, then the metal atom or ion.
For neutral ligands like water, ammonia, carbon monoxide has a common name called aqua, ammine, carbonyl respectively.
We use Greek prefixes to designate the number of each type of ligand in the complex ion. Example- di, tri, tetra, etc.
After naming the ligands, we name the central metal atom. If the complex is a cation, the metal is named the same as the element name. and if the complex ion is an anion, the name of metal has a suffix-ate. But for a neutral complex, the name of metal is written as it is.
Following the name of the metal, we write the oxidation state of the metal in the complex as a Roman numeral in parentheses.
Let us now name the given complex \[M{n_3}{(CO)_{12}}\] . We can see that this complex has a carbonyl ligand (i.e., carbon monoxide) which is 12 in number. So, as per rule number 3 it can be written as dodecacarbonyl. The 12 carbonyl (CO) ligands surround the central metal atom. The central atom present is manganese which is three in number, so we can write it as triamantanes. The manganese metal has zero charge on it. Therefore, as per rule number 5 we can represent it in parenthesis after writing the metal name.
Now, using rule number 1 and 2 we can write the name as Dodecacarbonyltrimanganese (0). It has three Mn-Mn bonds, ten terminal carbonyls and two bridged carbonyls. Its structure can be drawn as
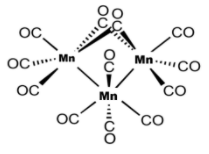
Hence, the correct option is (D).
Note:
The trimetallic compounds have a complicated structure and it is favoured by 18-electron configuration but with a relatively short Mn-Mn bond of 2.36 Angstrom. This is the characteristic feature of a formal Mn-Mn triple bond.
Complete step by step answer:
A complex is a substance in which a metal atom or ion in the centre is associated with a group of ligands. Ligands may be neutral or anionic. The coordination compounds are named as per IUPAC nomenclature rules.
In naming the complex ion, the name of the ligand is written first in alphabetical order, then the metal atom or ion.
For neutral ligands like water, ammonia, carbon monoxide has a common name called aqua, ammine, carbonyl respectively.
We use Greek prefixes to designate the number of each type of ligand in the complex ion. Example- di, tri, tetra, etc.
After naming the ligands, we name the central metal atom. If the complex is a cation, the metal is named the same as the element name. and if the complex ion is an anion, the name of metal has a suffix-ate. But for a neutral complex, the name of metal is written as it is.
Following the name of the metal, we write the oxidation state of the metal in the complex as a Roman numeral in parentheses.
Let us now name the given complex \[M{n_3}{(CO)_{12}}\] . We can see that this complex has a carbonyl ligand (i.e., carbon monoxide) which is 12 in number. So, as per rule number 3 it can be written as dodecacarbonyl. The 12 carbonyl (CO) ligands surround the central metal atom. The central atom present is manganese which is three in number, so we can write it as triamantanes. The manganese metal has zero charge on it. Therefore, as per rule number 5 we can represent it in parenthesis after writing the metal name.
Now, using rule number 1 and 2 we can write the name as Dodecacarbonyltrimanganese (0). It has three Mn-Mn bonds, ten terminal carbonyls and two bridged carbonyls. Its structure can be drawn as

Hence, the correct option is (D).
Note:
The trimetallic compounds have a complicated structure and it is favoured by 18-electron configuration but with a relatively short Mn-Mn bond of 2.36 Angstrom. This is the characteristic feature of a formal Mn-Mn triple bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




