
The correct decreasing order of \[p{K_a}\] in the given compound is:
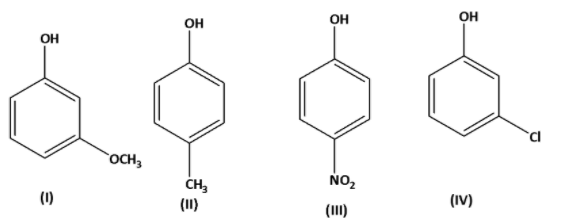
A. \[II > IV > I > III\]
B. \[IV > II > III > I\]
C. \[III > II > IV > I\]
D. \[IV > I > II > III\]

Answer
583.8k+ views
Hint: The term \[p{K_a}\] is equal to \[ - \log {K_a}\] , where \[{K_a}\] is the dissociation constant of acid. Now from this formula of \[p{K_a}\] it is clear that with increasing the value of dissociation constant the value of \[p{K_a}\] decreases and vice-versa. And with increasing the value of dissociation constant the acidity increases. Therefore, it can be said that with increases \[p{K_a}\] value acidity decreases and vice-versa.
Complete step by step answer:
Now phenol shows acidic behavior due to the delocalization of the lone pair of the oxygen into the benzene ring.
Higher the delocalization of the benzene ring higher is the positive charge density on the oxygen of phenol. Therefore, the bond polarity of \[O - H\] increases as well as acidity.
Now the acidity of phenol can be influenced by the presence of electron donating group or electron withdrawing group present in the benzene ring.
If an electron donating group is present then acidity of phenol increases as the electron deficiency increases as well as the bond polarity of the \[O - H\] bond.
If an electron withdrawing group is present the acidity of the phenol decreases as electron deficiency of the phenol decreases as well as bond polarity of the \[O - H\] bond.
Now from the question the electron withdrawing nature of the groups is,
\[ - N{O_2} > - OC{H_3} > - Cl > - C{H_3}\]
Therefore, the decreasing order of \[p{K_a}\] is \[II > IV > I > III\] .
Hence option A is correct.
Note:
Now to be an acidic hydrogen that hydrogen should be attached with a high electronegative group or electron withdrawing group. Higher the electronegativity of the group, higher will be the electron deficiency of the hydrogen, attached with that group as well as the acidity. Now in case or carbon the electronegativity varies with hybridization. With increasing the s character in the hybridization electronegativity of the carbon increases and vice-versa. Now the order of the electronegativity of the different hybridization of carbon is \[s{p^3} < s{p^2} < sp\] .Therefore, the order of the acidity of the hydrogen attached with these hybridized carbons is \[{C_{s{p^3}}} - H < {C_{s{p^2}}} - H < {C_{sp}} - H\] .
Complete step by step answer:
Now phenol shows acidic behavior due to the delocalization of the lone pair of the oxygen into the benzene ring.
Higher the delocalization of the benzene ring higher is the positive charge density on the oxygen of phenol. Therefore, the bond polarity of \[O - H\] increases as well as acidity.
Now the acidity of phenol can be influenced by the presence of electron donating group or electron withdrawing group present in the benzene ring.
If an electron donating group is present then acidity of phenol increases as the electron deficiency increases as well as the bond polarity of the \[O - H\] bond.
If an electron withdrawing group is present the acidity of the phenol decreases as electron deficiency of the phenol decreases as well as bond polarity of the \[O - H\] bond.
Now from the question the electron withdrawing nature of the groups is,
\[ - N{O_2} > - OC{H_3} > - Cl > - C{H_3}\]
Therefore, the decreasing order of \[p{K_a}\] is \[II > IV > I > III\] .
Hence option A is correct.
Note:
Now to be an acidic hydrogen that hydrogen should be attached with a high electronegative group or electron withdrawing group. Higher the electronegativity of the group, higher will be the electron deficiency of the hydrogen, attached with that group as well as the acidity. Now in case or carbon the electronegativity varies with hybridization. With increasing the s character in the hybridization electronegativity of the carbon increases and vice-versa. Now the order of the electronegativity of the different hybridization of carbon is \[s{p^3} < s{p^2} < sp\] .Therefore, the order of the acidity of the hydrogen attached with these hybridized carbons is \[{C_{s{p^3}}} - H < {C_{s{p^2}}} - H < {C_{sp}} - H\] .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




