
The correct acidity order of the following is:
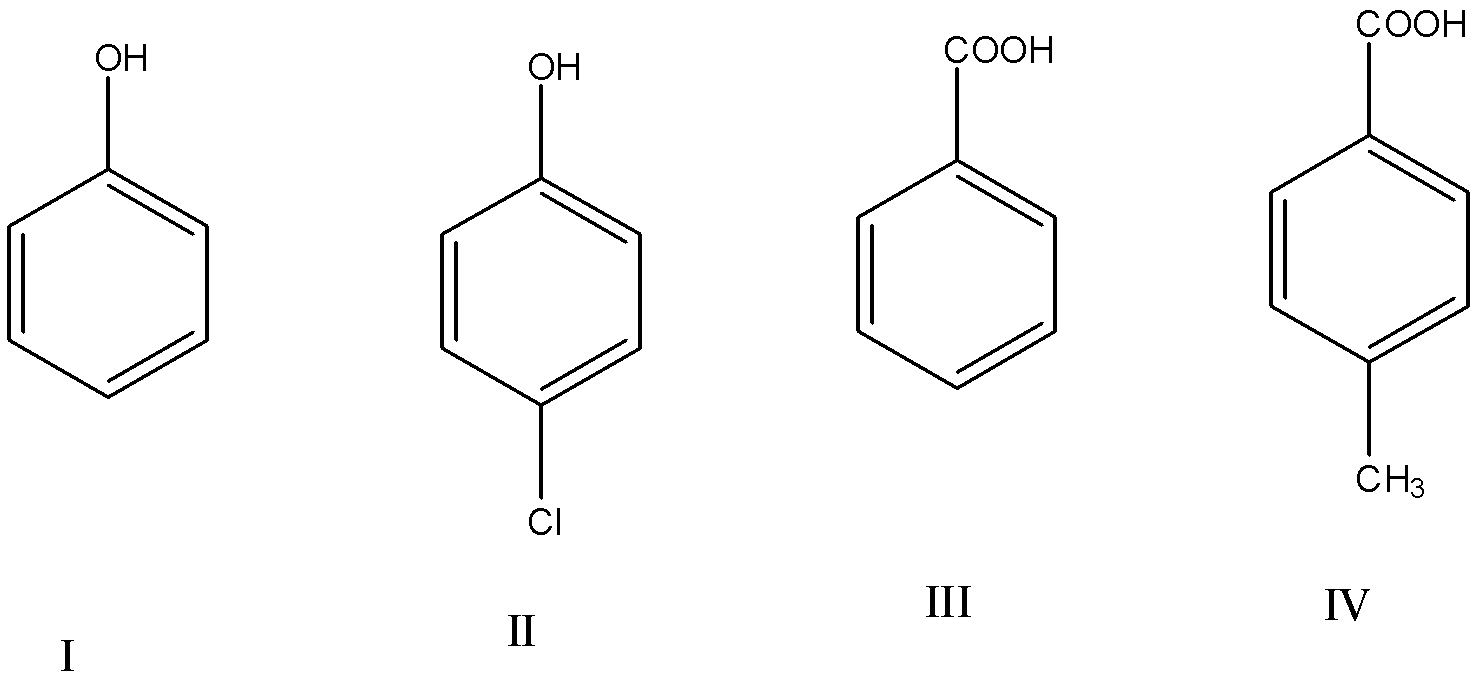
(a)- III>IV>II>I
(b)- IV>III>I>II
(c)- III>II>I>IV
(d)- II>III>IV>I

Answer
590.1k+ views
Hint: The acidity of phenol is due to the stability of phenoxide ion. When the electron-withdrawing group is present on the phenol the acidic character increases and the electron-donating group decreases the acidity. Carboxylic acids are more acidic than alcohols/phenols. When the electron-donating group is present on carboxylic acid the acidity decreases and when the electron-withdrawing group is present acidity increases.
Complete step by step answer:
Carboxylic acids are stronger acids than phenol because, in the resonating structures of phenoxide ion (when a hydrogen atom is removed from the O-H bond in phenol), the negative charge is on carbon which is less electronegative atom than oxygen. But in carboxylate ion (when a hydrogen atom is removed from COOH group), the negative charge is only on oxygen atom which has high electronegativity.
In both phenol and a carboxylic acid, when an electron-withdrawing group like $-N{{O}_{2}},-CN,-X(ha\log en)$, etc is attached to the molecule, the stability of the phenoxide ion and carboxylate ion increases by dispersing the negative charge which increases the acidity.
When an electron-donating group like $-C{{H}_{3}}$ is attached to the molecule, the stability of phenoxide ion and carboxylate ion decreases by intensifying the negative charge which decreases the acidity.
So, in III and IV, the acidity of IV is lesser than III, because the electron-donating group is present on IV.
In I and II, the acidity of II is greater than I, because the electron-withdrawing group is present on II.
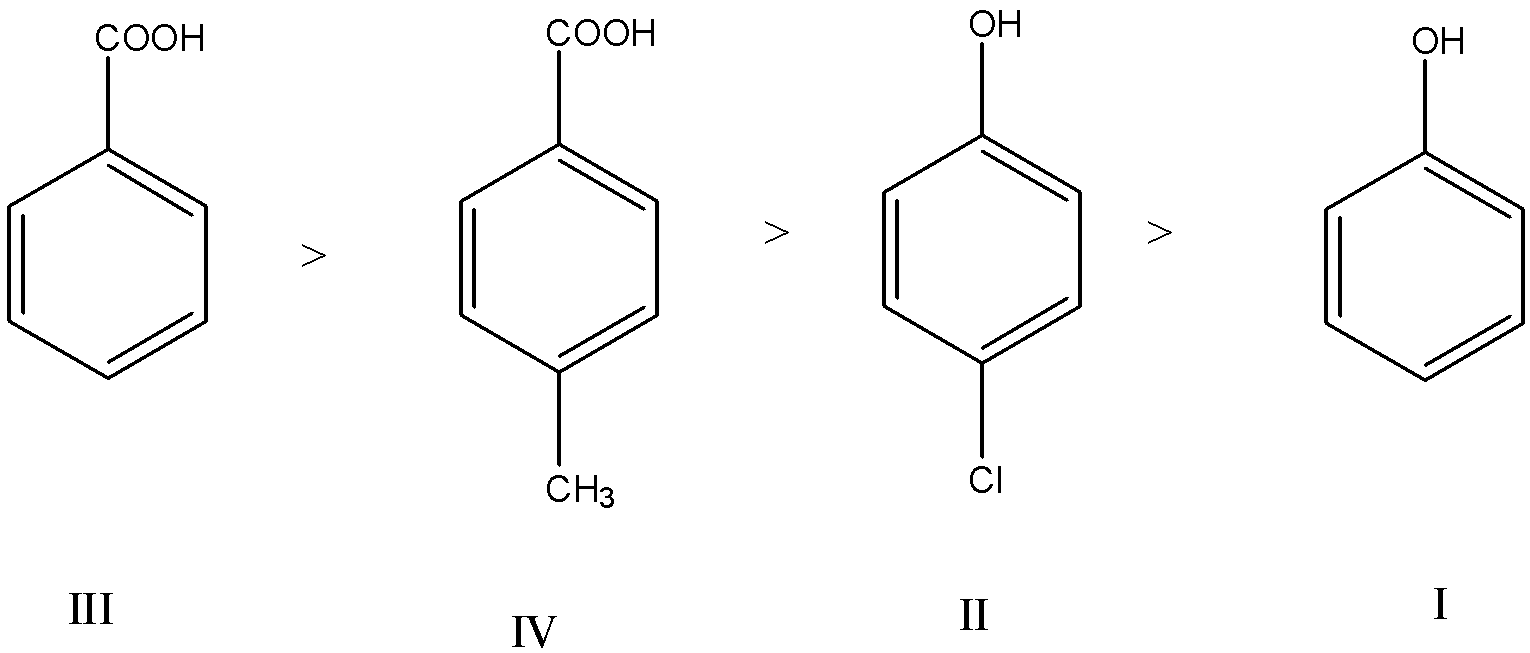
So, the stability order is III>IV>II>I
So, the correct answer is “Option A”.
Note: As the number of halogens or electron-withdrawing groups increases on the molecule, the acidity increases, and as the number of a methyl groups or electron-donating groups increases on the molecule, the acidity decreases.
Complete step by step answer:
Carboxylic acids are stronger acids than phenol because, in the resonating structures of phenoxide ion (when a hydrogen atom is removed from the O-H bond in phenol), the negative charge is on carbon which is less electronegative atom than oxygen. But in carboxylate ion (when a hydrogen atom is removed from COOH group), the negative charge is only on oxygen atom which has high electronegativity.
In both phenol and a carboxylic acid, when an electron-withdrawing group like $-N{{O}_{2}},-CN,-X(ha\log en)$, etc is attached to the molecule, the stability of the phenoxide ion and carboxylate ion increases by dispersing the negative charge which increases the acidity.
When an electron-donating group like $-C{{H}_{3}}$ is attached to the molecule, the stability of phenoxide ion and carboxylate ion decreases by intensifying the negative charge which decreases the acidity.
So, in III and IV, the acidity of IV is lesser than III, because the electron-donating group is present on IV.
In I and II, the acidity of II is greater than I, because the electron-withdrawing group is present on II.

So, the stability order is III>IV>II>I
So, the correct answer is “Option A”.
Note: As the number of halogens or electron-withdrawing groups increases on the molecule, the acidity increases, and as the number of a methyl groups or electron-donating groups increases on the molecule, the acidity decreases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




