
The coordination number of FCC structure for metals is $ 12 $ , since:
(A) Each atom touches $ 4 $ other in the same layer, $ 3 $ in layer above and $ 3 $ in layer below.
(B) Each atom touches $ 4 $ other in the same layer, $ 4 $ in layer above and $ 4 $ in layer below.
(C) Each atom touches $ 6 $ other in the same layer, $ 3 $ in layer above and $ 3 $ in layer below.
(D) Each atom touches $ 3 $ other in the same layer, $ 6 $ in layer above and $ 6 $ in layer below.
Answer
534.6k+ views
Hint: In FCC structure, one constituent atom is present at each corner of the cube and one atom at each center of its face. Coordination number of an atom is defined as the number of atoms, molecules or ions attached to it.
Complete answer:
In FCC structure, the atoms are present at each corner and each face center as shown in the following diagram.
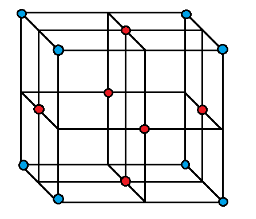
If we consider the face centred atom of the cube in FCC structure then we see that each face centred atom is surrounded by $ 4 $ corner atoms of its own layer and $ 4 $ face centred atoms of its adjacent upper layer as well as $ 4 $ adjacent lower layer. Therefore, the total number of atoms it is surrounded by is $ 4 + 4 + 4 = 12 $ . Hence, the coordination number of FCC structure for metals is $ 12 $ .
This proves that option B is correct.
Note:
Before attempting the question, try to imagine the structure of an FCC unit cell with precise location of the atoms. Also remember that there are $ 8 $ corner atoms and $ 6 $ central atoms in one FCC unit cell.
Complete answer:
In FCC structure, the atoms are present at each corner and each face center as shown in the following diagram.

If we consider the face centred atom of the cube in FCC structure then we see that each face centred atom is surrounded by $ 4 $ corner atoms of its own layer and $ 4 $ face centred atoms of its adjacent upper layer as well as $ 4 $ adjacent lower layer. Therefore, the total number of atoms it is surrounded by is $ 4 + 4 + 4 = 12 $ . Hence, the coordination number of FCC structure for metals is $ 12 $ .
This proves that option B is correct.
Note:
Before attempting the question, try to imagine the structure of an FCC unit cell with precise location of the atoms. Also remember that there are $ 8 $ corner atoms and $ 6 $ central atoms in one FCC unit cell.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




