
The compound which can be used to prepare iodoform is:
A.Methyl iodide
B.Acetone
C.Propionaldehyde
D.Acetic acid
Answer
594.9k+ views
Hint:Iodoform or triiodomethane is an organic compound with the formula ${\text{CH}}{{\text{I}}_{\text{3}}}$ and it is analogous to chloroform.
Complete step by step answer:
Iodoform can be prepared by the iodoform reaction. The iodoform reaction refers to the chemical reaction in which a methyl ketone is subjected to oxidation by allowing it to react with aqueous sodium hydroxide $\left( {{\text{NaOH}}} \right)$ and iodine $\left( {{{\text{I}}_{\text{2}}}} \right)$ to form a carboxylate. The iodoform reaction also produces a yellow solid which precipitates from the mixture. This yellow solid produced from the iodoform reaction is called iodoform. Since acetone is a methyl ketone, it can be used to prepare iodoform.
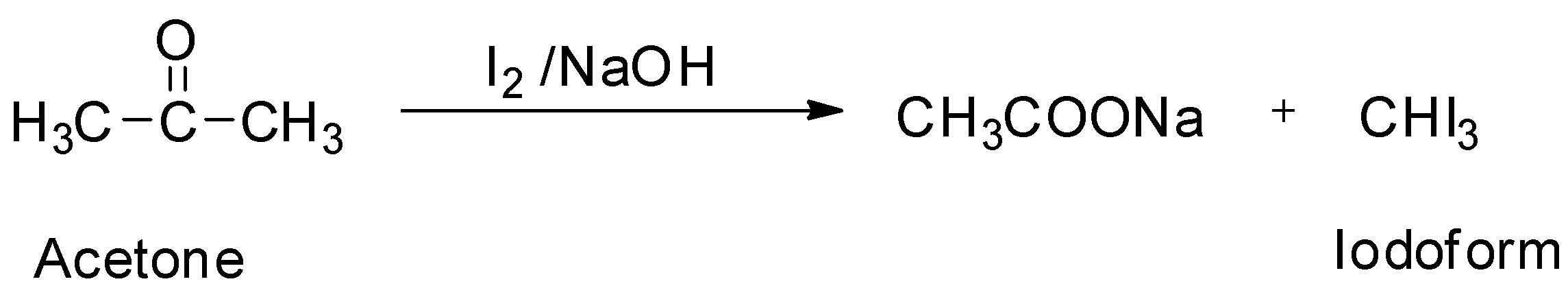
So ,option B is correct.
The other three options do not define a methyl ketone and so they are incorrect.
Additional information: (1) Iodoform can also be prepared from some other organic compounds like ethanol $\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}} \right)$, acetaldehyde $\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}} \right)$ and secondary alcohols (${\text{C}}{{\text{H}}_{\text{3}}}{\text{CHROH}}$where R represents an alkyl or aryl group) in place of a methyl ketone. Iodoform is synthesized by treating any of these four compounds with iodine $\left( {{{\text{I}}_{\text{2}}}} \right)$ and sodium hydroxide $\left( {{\text{NaOH}}} \right)$ in the haloform reaction.
(2)Iodoform is often used as a disinfectant, mainly in hospitals. It was also earlier used in medicine for treating wounds as a healing and antiseptic dressing.
(3)A chemical test called iodoform test is used to check the presence of a methyl ketone moiety in an unknown substance. The presence of methyl ketone moiety means the presence of carbonyl compounds having the structure ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COR}}$ or alcohols having the structure ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CHROH}}$ where R is an alkyl or aryl group. The pale yellow precipitate of iodoform produced in this test confirms the presence of methyl ketone. The only aldehyde which gives a positive iodoform test is acetaldehyde since it contains a ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CO - }}$ group.
Note:
Acetone is a methyl ketone since it has the structure ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COR}}$ where R is a methyl group.
Complete step by step answer:
Iodoform can be prepared by the iodoform reaction. The iodoform reaction refers to the chemical reaction in which a methyl ketone is subjected to oxidation by allowing it to react with aqueous sodium hydroxide $\left( {{\text{NaOH}}} \right)$ and iodine $\left( {{{\text{I}}_{\text{2}}}} \right)$ to form a carboxylate. The iodoform reaction also produces a yellow solid which precipitates from the mixture. This yellow solid produced from the iodoform reaction is called iodoform. Since acetone is a methyl ketone, it can be used to prepare iodoform.

So ,option B is correct.
The other three options do not define a methyl ketone and so they are incorrect.
Additional information: (1) Iodoform can also be prepared from some other organic compounds like ethanol $\left( {{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}} \right)$, acetaldehyde $\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}} \right)$ and secondary alcohols (${\text{C}}{{\text{H}}_{\text{3}}}{\text{CHROH}}$where R represents an alkyl or aryl group) in place of a methyl ketone. Iodoform is synthesized by treating any of these four compounds with iodine $\left( {{{\text{I}}_{\text{2}}}} \right)$ and sodium hydroxide $\left( {{\text{NaOH}}} \right)$ in the haloform reaction.
(2)Iodoform is often used as a disinfectant, mainly in hospitals. It was also earlier used in medicine for treating wounds as a healing and antiseptic dressing.
(3)A chemical test called iodoform test is used to check the presence of a methyl ketone moiety in an unknown substance. The presence of methyl ketone moiety means the presence of carbonyl compounds having the structure ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COR}}$ or alcohols having the structure ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CHROH}}$ where R is an alkyl or aryl group. The pale yellow precipitate of iodoform produced in this test confirms the presence of methyl ketone. The only aldehyde which gives a positive iodoform test is acetaldehyde since it contains a ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CO - }}$ group.
Note:
Acetone is a methyl ketone since it has the structure ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COR}}$ where R is a methyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




