
The compound that will not give iodoform on treatment with alkali and iodine is
A.Acetone
B.Ethanol
C.Diethyl ketone
D.Isopropyl alcohol
Answer
502.2k+ views
Hint: we have to remember that iodoform test is utilized to check the presence of carbonyl mixtures with the design $R - CO - C{H_3}$ or alcohols with the construction $R - CH\left( {OH} \right) - C{H_3}$ in a given obscure substance. The response of iodine, a base and a methyl ketone gives a yellow acceleration alongside a clean smell. It additionally tests positive for a couple of explicit auxiliary alcohols that contain no less than one methyl bunch in the alpha position.
Complete answer:
We have to know that an iodoform Test can be utilized to recognize the presence of carbonyl mixtures in alcohols. The response of Iodine alongside the base with methyl ketones brings about the presence of an exceptionally light yellow hasten of triiodomethane.
At the point when Iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or an optional liquor with a methyl bunch in the alpha position, a light yellow accelerant of iodoform or tri-iodomethane is framed. It tends to be utilized to recognize aldehydes or ketones. Assuming an aldehyde gives a positive iodoform test, it should be acetaldehyde since it is the lone aldehyde with a $C{H_3}C = O$ bunch. Given beneath are a couple of model responses for positive iodoform tests.
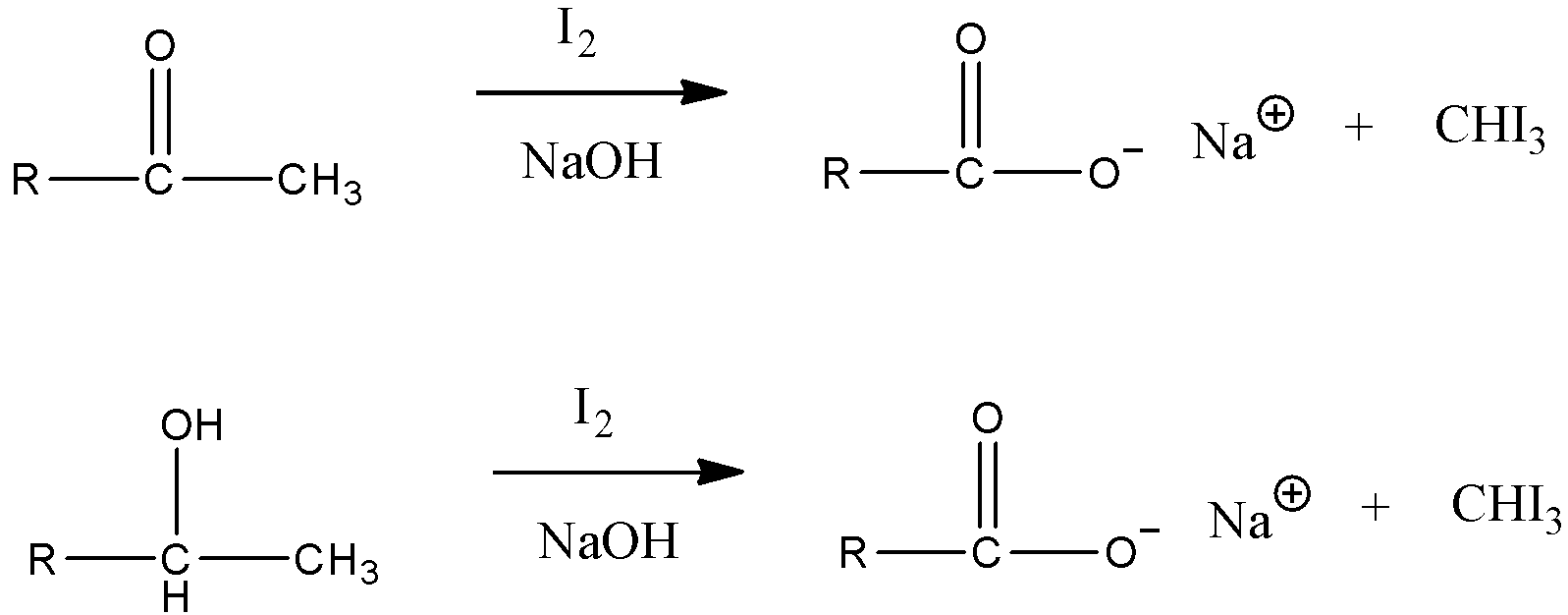
Therefore, the correct option is (C) diethyl ketone.
Note:
We have to know that, the methyl ketone response of iodine and base is dependable to such an extent that the iodoform test is utilized to test for methyl ketone presence. This is likewise the situation while looking for alpha-position delicate optional alcohols containing something like one methyl bunch.
Complete answer:
We have to know that an iodoform Test can be utilized to recognize the presence of carbonyl mixtures in alcohols. The response of Iodine alongside the base with methyl ketones brings about the presence of an exceptionally light yellow hasten of triiodomethane.
At the point when Iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or an optional liquor with a methyl bunch in the alpha position, a light yellow accelerant of iodoform or tri-iodomethane is framed. It tends to be utilized to recognize aldehydes or ketones. Assuming an aldehyde gives a positive iodoform test, it should be acetaldehyde since it is the lone aldehyde with a $C{H_3}C = O$ bunch. Given beneath are a couple of model responses for positive iodoform tests.

Therefore, the correct option is (C) diethyl ketone.
Note:
We have to know that, the methyl ketone response of iodine and base is dependable to such an extent that the iodoform test is utilized to test for methyl ketone presence. This is likewise the situation while looking for alpha-position delicate optional alcohols containing something like one methyl bunch.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




