
The compound $ [PtC{l_2}{(N{H_3})_2}] $ can form:
(A) Geometrical isomers
(B) Coordination isomers
(C) Linkage isomers
(D) Optical isomers
Answer
514.2k+ views
Hint :The compound $ [PtC{l_2}{(N{H_3})_2}] $ is a coordination compound with Pt as the central metal atom. A coordination compound consists of a central metal ion bound to a group of atoms or ions known as ligands. It may also contain a counter ion. Here, Pt is bound to Cl and $ N{H_3} $ which are anionic and neutral ligands respectively.
Complete Step By Step Answer:
The IUPAC name of the given compound $ [PtC{l_2}{(N{H_3})_2}] $ is diamminedichloroplatinum(II). Platinum is in $ + 2 $ oxidation state and the complex will have a square planar geometry.
Isomers are compounds with the same molecular formula but different structural formula. In coordination complexes, isomerism is mainly divided into structural isomerism and stereoisomerism. Among the given four isomers, geometrical and optical isomers belong to stereo isomerism and the other two belong to structural isomerism.
Coordination isomerism is observed in compounds having both cationic and anionic complex ions. The isomers are formed when the ligands are interchanged between the cationic and anionic ions.
Linkage isomerism exists in the presence of ambidentate ligands. Depending on the donor atom of the ligand linked to the metal, isomers are formed.
Geometrical isomers have the same ligands in their coordination sphere, but the relative position of the ligands around the central metal atom will be different.
Optical isomers happen when a given molecule is not superimposable with its mirror image.
Therefore, $ [PtC{l_2}{(N{H_3})_2}] $ exhibits geometrical isomerism. It is a square planar complex and when the similar ligands are adjacent to each other ( $ {90^ \circ } $ apart), it is called cis-isomer and when they are opposite to each other ( $ {180^ \circ } $ apart), it is called as trans isomer.
The cis and trans geometrical isomers of $ [PtC{l_2}{(N{H_3})_2}] $ are:
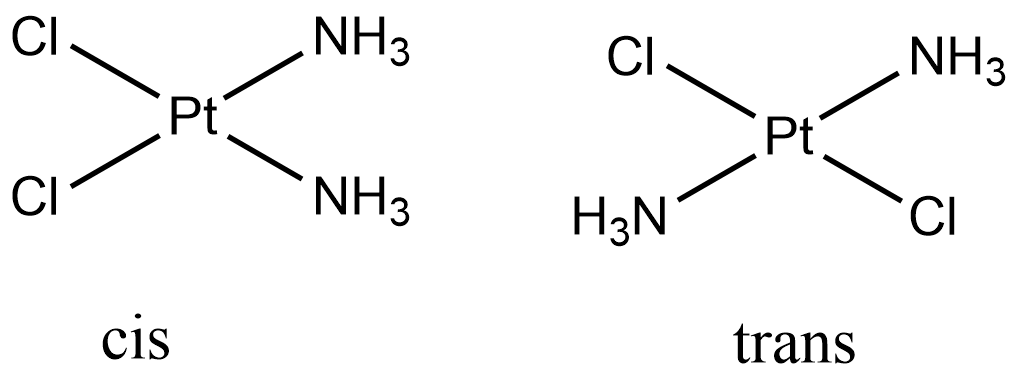
The right option is (A) Geometrical isomers.
Note :
In $ [PtC{l_2}{(N{H_3})_2}] $ , chloride is an anionic ligand with $ ( - 1) $ charge and $ N{H_3} $ is a neutral ligand (charge is zero). Therefore, they are named chloride and amine respectively. Since there are two ammine and chlorido ligands, a prefix “di” is added before their names. After balancing the oxidation states of ligands, we get the oxidation state of Pt as $ + 2 $ . Thus IUPAC name of the complex becomes diamminechloridoplatinum(II). It is a $ M{a_2}{b_2} $ type square planar complex having cis and trans geometrical isomers (M=metal, a $ \& $ b =ligands) .
Complete Step By Step Answer:
The IUPAC name of the given compound $ [PtC{l_2}{(N{H_3})_2}] $ is diamminedichloroplatinum(II). Platinum is in $ + 2 $ oxidation state and the complex will have a square planar geometry.
Isomers are compounds with the same molecular formula but different structural formula. In coordination complexes, isomerism is mainly divided into structural isomerism and stereoisomerism. Among the given four isomers, geometrical and optical isomers belong to stereo isomerism and the other two belong to structural isomerism.
Coordination isomerism is observed in compounds having both cationic and anionic complex ions. The isomers are formed when the ligands are interchanged between the cationic and anionic ions.
Linkage isomerism exists in the presence of ambidentate ligands. Depending on the donor atom of the ligand linked to the metal, isomers are formed.
Geometrical isomers have the same ligands in their coordination sphere, but the relative position of the ligands around the central metal atom will be different.
Optical isomers happen when a given molecule is not superimposable with its mirror image.
Therefore, $ [PtC{l_2}{(N{H_3})_2}] $ exhibits geometrical isomerism. It is a square planar complex and when the similar ligands are adjacent to each other ( $ {90^ \circ } $ apart), it is called cis-isomer and when they are opposite to each other ( $ {180^ \circ } $ apart), it is called as trans isomer.
The cis and trans geometrical isomers of $ [PtC{l_2}{(N{H_3})_2}] $ are:

The right option is (A) Geometrical isomers.
Note :
In $ [PtC{l_2}{(N{H_3})_2}] $ , chloride is an anionic ligand with $ ( - 1) $ charge and $ N{H_3} $ is a neutral ligand (charge is zero). Therefore, they are named chloride and amine respectively. Since there are two ammine and chlorido ligands, a prefix “di” is added before their names. After balancing the oxidation states of ligands, we get the oxidation state of Pt as $ + 2 $ . Thus IUPAC name of the complex becomes diamminechloridoplatinum(II). It is a $ M{a_2}{b_2} $ type square planar complex having cis and trans geometrical isomers (M=metal, a $ \& $ b =ligands) .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




