
The compound formed when calcium acetate and calcium formate is dry distilled.
A. Acetone
B. Acetaldehyde
C. Benzaldehyde
D. Acetophenone
Answer
586.2k+ views
Hint: Revise the concepts of preparation of organic compounds like aldehydes and ketones. Start by writing the structures of calcium acetate and calcium formate. Both are calcium salts of carboxylic acids. If dry distillation involves removal of calcium carbonate, write the reaction and identify which product will be formed to get the answer.
Complete step by step answer:
First let’s see what calcium acetate and calcium formate are.
Calcium acetate is calcium salt of acetic acid. It has the formula ${{\left( C{{H}_{3}}COO \right)}_{2}}Ca$. Its structure is as follows:
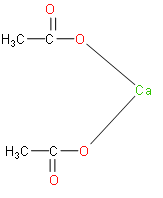
The above structure is of calcium acetate.
Calcium formate is the calcium salt of formic acid. It has the formula, ${{\left( HCOO \right)}_{2}}Ca$. The structure of calcium formate is as follows:
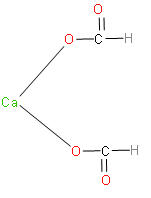
Now, let’s write the reaction which takes place when calcium formate reacts with calcium acetate.
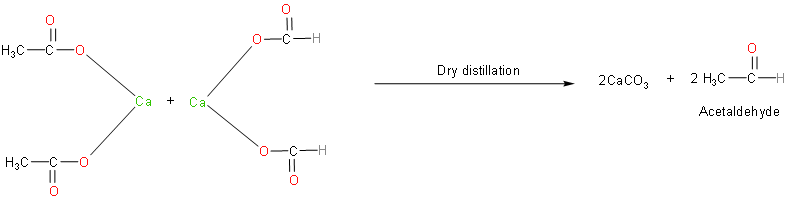
Calcium formate and calcium acetate produce acetaldehyde on dry distillation with calcium carbonate as the by-product. Acetaldehyde (ethanal) is an aldehyde that is highly reactive and toxic. We should know that it is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry.
So, the correct answer is “Option B”.
Note: Acetaldehyde is generally produced by the oxidation of ethylene by the Wacker process, which involves oxidation of ethylene using a homogeneous palladium/copper system.
We find acetaldehyde naturally in coffee, bread, and ripe fruit. Remember that acetaldehyde is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption.
Complete step by step answer:
First let’s see what calcium acetate and calcium formate are.
Calcium acetate is calcium salt of acetic acid. It has the formula ${{\left( C{{H}_{3}}COO \right)}_{2}}Ca$. Its structure is as follows:

The above structure is of calcium acetate.
Calcium formate is the calcium salt of formic acid. It has the formula, ${{\left( HCOO \right)}_{2}}Ca$. The structure of calcium formate is as follows:

Now, let’s write the reaction which takes place when calcium formate reacts with calcium acetate.

Calcium formate and calcium acetate produce acetaldehyde on dry distillation with calcium carbonate as the by-product. Acetaldehyde (ethanal) is an aldehyde that is highly reactive and toxic. We should know that it is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry.
So, the correct answer is “Option B”.
Note: Acetaldehyde is generally produced by the oxidation of ethylene by the Wacker process, which involves oxidation of ethylene using a homogeneous palladium/copper system.
We find acetaldehyde naturally in coffee, bread, and ripe fruit. Remember that acetaldehyde is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




