
The chemical name of lunar caustic is:
A. sodium sulphate
B. silver nitrate
C. calcium carbonate
D. mercuric chloride
Answer
576k+ views
Hint: The compound is used as an antiseptic in medicinal chemistry. It has a molar mass of about 170 grams per mole. Moreover, the compound is ionic in nature and readily dissolves into water.
Complete step by step solution:
-Silver nitrate is a caustic compound, and is used as an antiseptic , as a reagent for preparing salts industrially, as well as acting as a reagent in analytical chemistry. Silver nitrate has the chemical formula of $AgN{{O}_{3}}$ and has a molar mass of 170 gram per mole. A solution is prepared, where 0.01 percent to 10 percent silver nitrate solution is added in water. This solution acts as an antiseptic and can be used to heal wounds. Concentrations of 1 to 3 percent can be used to treat gonococcal bacteria in the eyes of newborn infants.
-Apart from the use of silver nitrate as antiseptic, it can be also used for preparation of other salts. They can be used to prepare the colloids present in medicine, which includes silver, as well as silver halides, which are used in photography. It can be also used to determine the volume of halides, cyanides as well as thiocyanates. In the preparation of silver nitrate, concentrated or dilute nitric acid is taken and large quantities of silver are dissolved in it.
\[\begin{align}
& 3Ag+4HN{{O}_{3}}(cold,dil)\to 3AgN{{O}_{3}}+2{{H}_{2}}O+NO \\
& Ag+2HN{{O}_{3}}(hot,conc)\to AgN{{O}_{3}}+{{H}_{2}}O+N{{O}_{2}} \\
\end{align}\]
$AgN{{O}_{3}}$is ionic in nature and it dissolves in water to form the silver cation$A{{g}^{+}}$and the nitrate anion $N{{O}_{3}}^{-}$. Here is how the silver nitrate looks like:
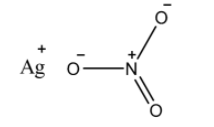
Note: When silver nitrate is heated at very high temperature, then it loses the oxygen molecule, and silver nitride is formed. On further heating, silver is formed. It is also to be noted that the nitrate anion undergoes resonance.
Complete step by step solution:
-Silver nitrate is a caustic compound, and is used as an antiseptic , as a reagent for preparing salts industrially, as well as acting as a reagent in analytical chemistry. Silver nitrate has the chemical formula of $AgN{{O}_{3}}$ and has a molar mass of 170 gram per mole. A solution is prepared, where 0.01 percent to 10 percent silver nitrate solution is added in water. This solution acts as an antiseptic and can be used to heal wounds. Concentrations of 1 to 3 percent can be used to treat gonococcal bacteria in the eyes of newborn infants.
-Apart from the use of silver nitrate as antiseptic, it can be also used for preparation of other salts. They can be used to prepare the colloids present in medicine, which includes silver, as well as silver halides, which are used in photography. It can be also used to determine the volume of halides, cyanides as well as thiocyanates. In the preparation of silver nitrate, concentrated or dilute nitric acid is taken and large quantities of silver are dissolved in it.
\[\begin{align}
& 3Ag+4HN{{O}_{3}}(cold,dil)\to 3AgN{{O}_{3}}+2{{H}_{2}}O+NO \\
& Ag+2HN{{O}_{3}}(hot,conc)\to AgN{{O}_{3}}+{{H}_{2}}O+N{{O}_{2}} \\
\end{align}\]
$AgN{{O}_{3}}$is ionic in nature and it dissolves in water to form the silver cation$A{{g}^{+}}$and the nitrate anion $N{{O}_{3}}^{-}$. Here is how the silver nitrate looks like:

Note: When silver nitrate is heated at very high temperature, then it loses the oxygen molecule, and silver nitride is formed. On further heating, silver is formed. It is also to be noted that the nitrate anion undergoes resonance.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




