
The CFSE for ${\left[ {{\text{CoC}}{{\text{l}}_6}} \right]^{4 - }}$ complex is $18000\,{\text{c}}{{\text{m}}^{ - 1}}$. The $ $ for ${\left[ {{\text{CoC}}{{\text{l}}_4}} \right]^{2 -}}$ will be
A. $18000\,{\text{c}}{{\text{m}}^{ - 1}}$
B. $16000\,{\text{c}}{{\text{m}}^{ - 1}}$
C. $6000\,{\text{c}}{{\text{m}}^{ - 1}}$
D. $2000\,{\text{c}}{{\text{m}}^{ - 1}}$
Answer
581.4k+ views
Hint: The octahedral crystal field splitting is equal to the $4/9$ of octahedral crystal field splitting. The octahedral crystal field splitting is larger than the tetrahedral crystal field splitting.
The following formula can be used-
$
{{\text{ }}_{{\text{td}}}}\,{\text{ = }}\,\dfrac{{\text{4}}}{{\,{\text{9}}}}{{\text{ }}_{{\text{oh}}}}
$
Step by step answer: he octahedral field splitting is represented as follows:
Valence electronic configuration of ${\text{C}}{{\text{o}}^{2 + }}$ = ${\text{3}}{{\text{d}}^7}$
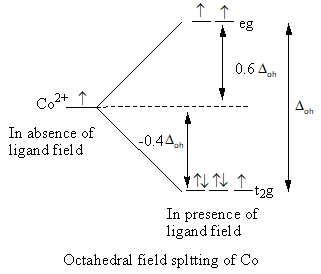
The tetrahedral field splitting is represented as follows:
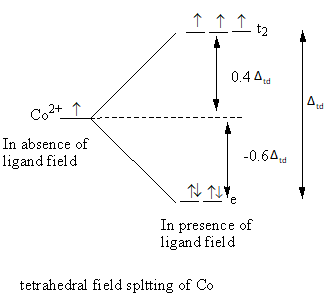
The relation between the energy difference of tetrahedral and octahedral field is as follows:
\[{{\text{ }}_{{\text{td}}}}\,{\text{ = }}\,\dfrac{{\text{4}}}{{\,{\text{9}}}}{{\text{ }}_{{\text{oh}}}}\]
Where,
\[{{\text{ }}_{{\text{td}}}}\]is the tetrahedral field splitting energy.
${ _{{\text{oh}}}}$ is the octahedral field splitting energy.
In the octahedral complex, six ligands split the energy level of the metal and the tetrahedral complex four ligands split the energy level of the metal, so the value of octahedral crystal field splitting is larger than the tetrahedral crystal field splitting.
Substitute $18000\,{\text{c}}{{\text{m}}^{ - 1}}$ for ${ _{{\text{oh}}}}$.
\[{{\text{ }}_{{\text{td}}}}\,{\text{ = }}\,\dfrac{{\text{4}}}{{\,{\text{9}}}} \times 18000\,{\text{c}}{{\text{m}}^{ - 1}}\]
\[{{\text{ }}_{{\text{td}}}}\,{\text{ = }}\,8000\,{\text{c}}{{\text{m}}^{ - 1}}\]
So, the tetrahedral field splitting energy for ${\left[ {{\text{CoC}}{{\text{l}}_4}} \right]^{2 - }}$complex is $8000\,{\text{c}}{{\text{m}}^{ - 1}}$.
So, option (A), (B) and (D) are incorrect.
Therefore, option (C) $8000\,{\text{c}}{{\text{m}}^{ - 1}}$ is correct.
Additional information: The ${ _{{\text{oh}}}}$ per ligand is determined by dividing the ${ _{{\text{oh}}}}$ by six and \[{{\text{ }}_{{\text{td}}}}\] per ligand is determined by dividing the \[{{\text{ }}_{{\text{td}}}}\] by four.
Note: Both the $ $values for octahedral as well as tetrahedral should be in the same unit. As the octahedral crystal field splitting is always larger than the tetrahedral crystal field splitting so, here, the answer of \[{{\text{ }}_{{\text{td}}}}\] will be less than$18000\,{\text{c}}{{\text{m}}^{ - 1}}$. For the calculation of ${ _{{\text{oh}}}}$ from the given \[{{\text{ }}_{{\text{td}}}}\], the formula used will be \[\,{{\text{ }}_{{\text{oh}}}}{\text{ = }}\,\dfrac{9}{{\,4}}{{\text{ }}_{{\text{td}}}}\].
The following formula can be used-
$
{{\text{ }}_{{\text{td}}}}\,{\text{ = }}\,\dfrac{{\text{4}}}{{\,{\text{9}}}}{{\text{ }}_{{\text{oh}}}}
$
Step by step answer: he octahedral field splitting is represented as follows:
Valence electronic configuration of ${\text{C}}{{\text{o}}^{2 + }}$ = ${\text{3}}{{\text{d}}^7}$

The tetrahedral field splitting is represented as follows:

The relation between the energy difference of tetrahedral and octahedral field is as follows:
\[{{\text{ }}_{{\text{td}}}}\,{\text{ = }}\,\dfrac{{\text{4}}}{{\,{\text{9}}}}{{\text{ }}_{{\text{oh}}}}\]
Where,
\[{{\text{ }}_{{\text{td}}}}\]is the tetrahedral field splitting energy.
${ _{{\text{oh}}}}$ is the octahedral field splitting energy.
In the octahedral complex, six ligands split the energy level of the metal and the tetrahedral complex four ligands split the energy level of the metal, so the value of octahedral crystal field splitting is larger than the tetrahedral crystal field splitting.
Substitute $18000\,{\text{c}}{{\text{m}}^{ - 1}}$ for ${ _{{\text{oh}}}}$.
\[{{\text{ }}_{{\text{td}}}}\,{\text{ = }}\,\dfrac{{\text{4}}}{{\,{\text{9}}}} \times 18000\,{\text{c}}{{\text{m}}^{ - 1}}\]
\[{{\text{ }}_{{\text{td}}}}\,{\text{ = }}\,8000\,{\text{c}}{{\text{m}}^{ - 1}}\]
So, the tetrahedral field splitting energy for ${\left[ {{\text{CoC}}{{\text{l}}_4}} \right]^{2 - }}$complex is $8000\,{\text{c}}{{\text{m}}^{ - 1}}$.
So, option (A), (B) and (D) are incorrect.
Therefore, option (C) $8000\,{\text{c}}{{\text{m}}^{ - 1}}$ is correct.
Additional information: The ${ _{{\text{oh}}}}$ per ligand is determined by dividing the ${ _{{\text{oh}}}}$ by six and \[{{\text{ }}_{{\text{td}}}}\] per ligand is determined by dividing the \[{{\text{ }}_{{\text{td}}}}\] by four.
Note: Both the $ $values for octahedral as well as tetrahedral should be in the same unit. As the octahedral crystal field splitting is always larger than the tetrahedral crystal field splitting so, here, the answer of \[{{\text{ }}_{{\text{td}}}}\] will be less than$18000\,{\text{c}}{{\text{m}}^{ - 1}}$. For the calculation of ${ _{{\text{oh}}}}$ from the given \[{{\text{ }}_{{\text{td}}}}\], the formula used will be \[\,{{\text{ }}_{{\text{oh}}}}{\text{ = }}\,\dfrac{9}{{\,4}}{{\text{ }}_{{\text{td}}}}\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




