
The Carnot cycle of a reversible heat engine consists of:
A. One isothermal and two adiabatic processes.
B. Two isothermal and one adiabatic processes.
C. Two isothermal and two adiabatic processes.
D. Two isobaric and two isothermal processes.
E. Two isochoric and two adiabatic processes.
Answer
596.4k+ views
Hint: In an isothermal process, temperature remains constant. In an isobaric process, the pressure remains constant. In an isochoric process, the volume remains constant. In an adiabatic process, the heat transfer is zero.
Complete step-by-step answer:
This cycle is one of the foundations of the second law of thermodynamics, and Carnot is often considered the father of thermodynamics. He was one of the pioneers who first determined an idealistic way of converting heat energy into work done. Carnot cycle is one of the most efficient heat engines.
Carnot cycle consists of the following four processes:
I. The gas goes through an isothermal expansion at a high temperature. In this process the gas takes ${{q}_{in}}$ amount of heat from the surrounding and does ${{w}_{1}}$ amount of work on the surrounding.
II. The gas then undergoes a reversible adiabatic expansion. Hence, the temperature of the gas comes down to a lower temperature ${{T}_{low}}$.
III. Then the gas is compressed isothermally at ${{T}_{low}}$ temperature. In this process, the gas loses ${{q}_{out}}$ amount of heat, and surroundings do work on the gas.
IV. Now the gas goes through a reversible adiabatic compression which makes the temperature rise up to ${{T}_{high}}$.
The following diagram shows the P-V diagram of the Carnot’s cycle.
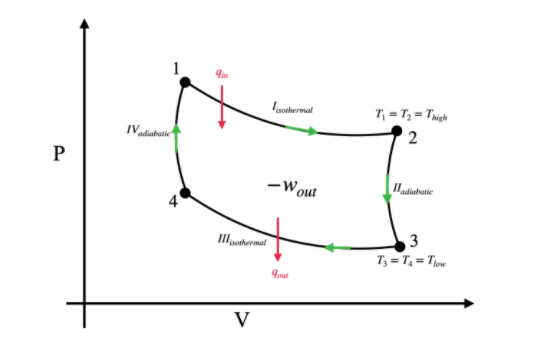
Hence, Carnot’s cycle consists of two isothermal and two adiabatic processes.
The correct option is - (C).
Note:We can calculate the temperature change, work done, and heat change to determine the efficiency of the Carnot’s engine. Finally, the expression depends on the temperatures of the cycle. The efficiency is given by,
$\dfrac{{{T}_{high}}-{{T}_{low}}}{{{T}_{high}}}\times 100$
Where,
${{T}_{high}}$ is the high temperature
${{T}_{low}}$ is the low temperature
Complete step-by-step answer:
This cycle is one of the foundations of the second law of thermodynamics, and Carnot is often considered the father of thermodynamics. He was one of the pioneers who first determined an idealistic way of converting heat energy into work done. Carnot cycle is one of the most efficient heat engines.
Carnot cycle consists of the following four processes:
I. The gas goes through an isothermal expansion at a high temperature. In this process the gas takes ${{q}_{in}}$ amount of heat from the surrounding and does ${{w}_{1}}$ amount of work on the surrounding.
II. The gas then undergoes a reversible adiabatic expansion. Hence, the temperature of the gas comes down to a lower temperature ${{T}_{low}}$.
III. Then the gas is compressed isothermally at ${{T}_{low}}$ temperature. In this process, the gas loses ${{q}_{out}}$ amount of heat, and surroundings do work on the gas.
IV. Now the gas goes through a reversible adiabatic compression which makes the temperature rise up to ${{T}_{high}}$.
The following diagram shows the P-V diagram of the Carnot’s cycle.

Hence, Carnot’s cycle consists of two isothermal and two adiabatic processes.
The correct option is - (C).
Note:We can calculate the temperature change, work done, and heat change to determine the efficiency of the Carnot’s engine. Finally, the expression depends on the temperatures of the cycle. The efficiency is given by,
$\dfrac{{{T}_{high}}-{{T}_{low}}}{{{T}_{high}}}\times 100$
Where,
${{T}_{high}}$ is the high temperature
${{T}_{low}}$ is the low temperature
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




