
The basic amino acids are:
(a)- Lysine, Arginine
(b)- Alanine, Glutamic acid
(c)- Proline, Valine
(d)- Alanine, Cysteine
Answer
542.1k+ views
Hint: There are three types of amino acids: acidic, neutral, and basic amino acids. Basic amino acids mean there is a higher number of amine groups as compared to the carboxylic acid group. The basic amino acids represented with one letter code are K, R, H, etc.
Complete step-by-step answer:We know that the amino acids are those compounds in which there is one acid group, i.e., carboxylic acid group, and one basic group, i.e., amine group. The acid group is represented with $-COOH$ and the basic group is represented by $-N{{H}_{2}}$. So, based on these groups we can classify the amino acids as acidic amino acids, neutral amino acids, and basic amino acids.
When the number of acid groups is more than the amino group in the same compound then it is called an acidic amino acid. When the number of an amino group and the carboxylic acid is the same then it is a neutral amino acid. When the number of amine groups is more than the acid groups in the same compound then it is a basic amino acid.
Some examples of basic amino acids are Lysine, Arginine, etc. The structure of Lysine is given below:
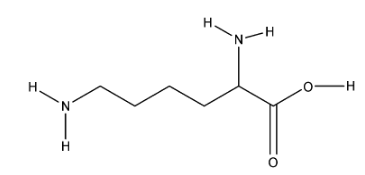
In this, there are 2 amine groups and 1 acid group. It is represented with K.
The structure of Arginine is given below:
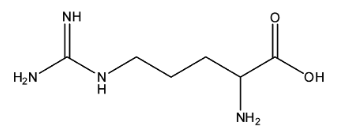
This structure also has more amine groups than the acid group. It is represented with R.
Therefore, the correct answer is an option (a)- Lysine, Arginine.
Note:Mostly the names of the acidic amino acids have acid in the suffix part like Aspartic acid, Glutamic acid because the number of acid groups is more than the amine groups.
Complete step-by-step answer:We know that the amino acids are those compounds in which there is one acid group, i.e., carboxylic acid group, and one basic group, i.e., amine group. The acid group is represented with $-COOH$ and the basic group is represented by $-N{{H}_{2}}$. So, based on these groups we can classify the amino acids as acidic amino acids, neutral amino acids, and basic amino acids.
When the number of acid groups is more than the amino group in the same compound then it is called an acidic amino acid. When the number of an amino group and the carboxylic acid is the same then it is a neutral amino acid. When the number of amine groups is more than the acid groups in the same compound then it is a basic amino acid.
Some examples of basic amino acids are Lysine, Arginine, etc. The structure of Lysine is given below:

In this, there are 2 amine groups and 1 acid group. It is represented with K.
The structure of Arginine is given below:

This structure also has more amine groups than the acid group. It is represented with R.
Therefore, the correct answer is an option (a)- Lysine, Arginine.
Note:Mostly the names of the acidic amino acids have acid in the suffix part like Aspartic acid, Glutamic acid because the number of acid groups is more than the amine groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




