
The average binding energy of the nucleon is
(A) $ 931\;MeV $
(B) $ 8.5\;MeV $
(C) $ 1.6\;MeV $
(D) $ 3\;MeV $
Answer
533.7k+ views
Hint : Binding energy of the nucleon can be defined as the energy released due to the mass difference in the formation of a nucleus. Binding energy per nucleon is the binding energy divided by the nucleon number. Average binding energy is a constant value.
Complete Step By Step Answer:
We know that the nucleus is made of protons and neutrons. This means, if we consider logically, then the mass of the nucleus should be equal to the sum of the mass of total protons and total neutrons.
However, it is proved from the mass spectroscopy experiments, that the mass of the nucleus is always less than the sum of the mass of the protons and neutrons in the free-state.
This difference in the masses is known as the mass defect, which can be calculated as,
$ \Delta M = (Z{m_p} + (A - Z){m_n}) - M $
Where, $ Z $ is the atomic number of the element, $ A $ is the atomic mass number of the element, $ {m_p} $ is the mass of the proton, $ {m_n} $ is the mass of the nucleus, and $ M $ is the mass of the nucleus obtained experimentally through mass spectroscopy.
We can have a better understanding of this mass defect by considering Einstein’s theory of relativity equation $ E = m{c^2} $
Thus, when the nucleons form a nucleus from the free-state, energy equivalent to this mass defect is released which is known as the binding energy of the nucleus $ {E_b} $ .
Binding energy can also be defined as the energy required to be provided to break the nucleus into individual constituents.
Now, the practically more used term, the binding energy per nucleon can be defined as the ratio of the binding energy and the atomic mass number or the total number of nucleons.
$ \therefore {E_{bn}} = \dfrac{{{E_b}}}{A} $
The plot for binding energy per nucleon of various elements can be shown as below,
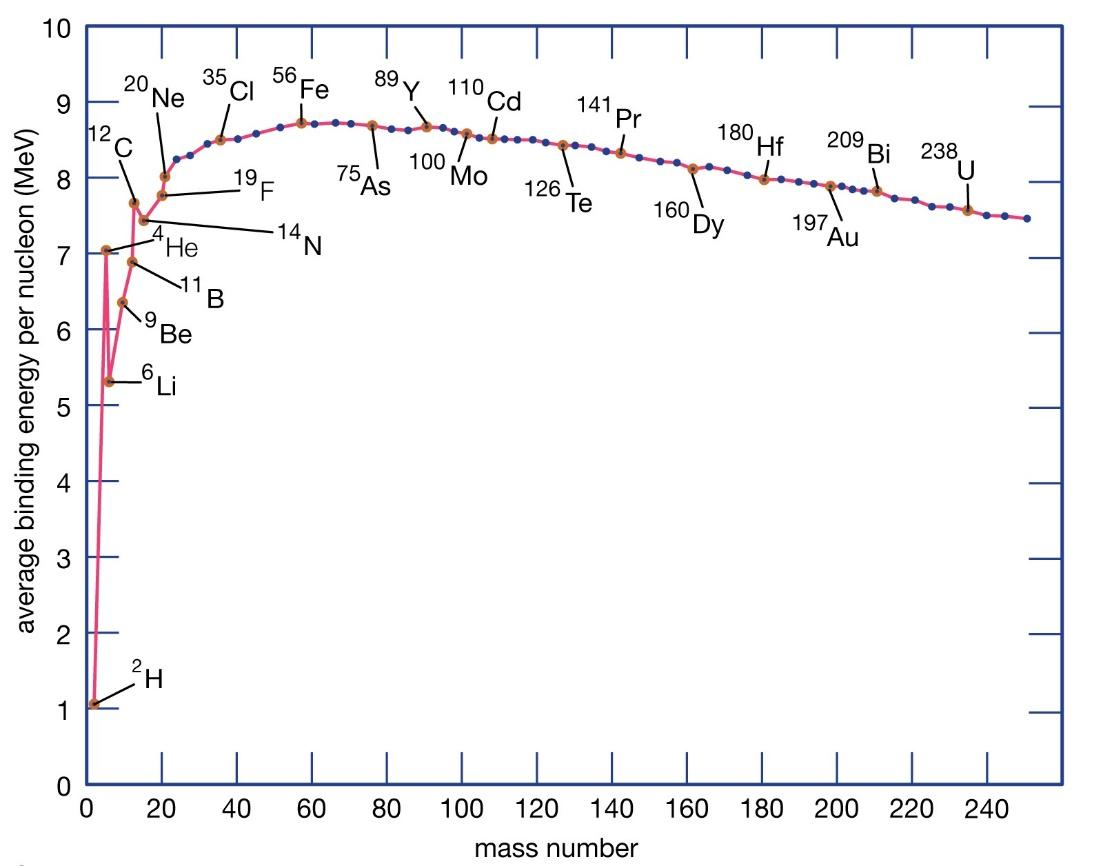
From the graph, we can understand that the maximum binding energy per nucleon is maximum for $ A = 56 $ .
And the average binding energy per nucleon from the graph can be deduced to be $ 8.5\;MeV $ .
Hence, the correct answer is Option $ (B) $ .
Note :
Here, we must know the difference between the mass-energy of a nucleon and nuclear binding energy per nucleon. Mass energy is the equivalence between mass and energy, and how they are interrelated. Mass energy of a nucleon is $ 931\;MeV $ .
Complete Step By Step Answer:
We know that the nucleus is made of protons and neutrons. This means, if we consider logically, then the mass of the nucleus should be equal to the sum of the mass of total protons and total neutrons.
However, it is proved from the mass spectroscopy experiments, that the mass of the nucleus is always less than the sum of the mass of the protons and neutrons in the free-state.
This difference in the masses is known as the mass defect, which can be calculated as,
$ \Delta M = (Z{m_p} + (A - Z){m_n}) - M $
Where, $ Z $ is the atomic number of the element, $ A $ is the atomic mass number of the element, $ {m_p} $ is the mass of the proton, $ {m_n} $ is the mass of the nucleus, and $ M $ is the mass of the nucleus obtained experimentally through mass spectroscopy.
We can have a better understanding of this mass defect by considering Einstein’s theory of relativity equation $ E = m{c^2} $
Thus, when the nucleons form a nucleus from the free-state, energy equivalent to this mass defect is released which is known as the binding energy of the nucleus $ {E_b} $ .
Binding energy can also be defined as the energy required to be provided to break the nucleus into individual constituents.
Now, the practically more used term, the binding energy per nucleon can be defined as the ratio of the binding energy and the atomic mass number or the total number of nucleons.
$ \therefore {E_{bn}} = \dfrac{{{E_b}}}{A} $
The plot for binding energy per nucleon of various elements can be shown as below,

From the graph, we can understand that the maximum binding energy per nucleon is maximum for $ A = 56 $ .
And the average binding energy per nucleon from the graph can be deduced to be $ 8.5\;MeV $ .
Hence, the correct answer is Option $ (B) $ .
Note :
Here, we must know the difference between the mass-energy of a nucleon and nuclear binding energy per nucleon. Mass energy is the equivalence between mass and energy, and how they are interrelated. Mass energy of a nucleon is $ 931\;MeV $ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




