
The appropriate reagent for the following transformation:
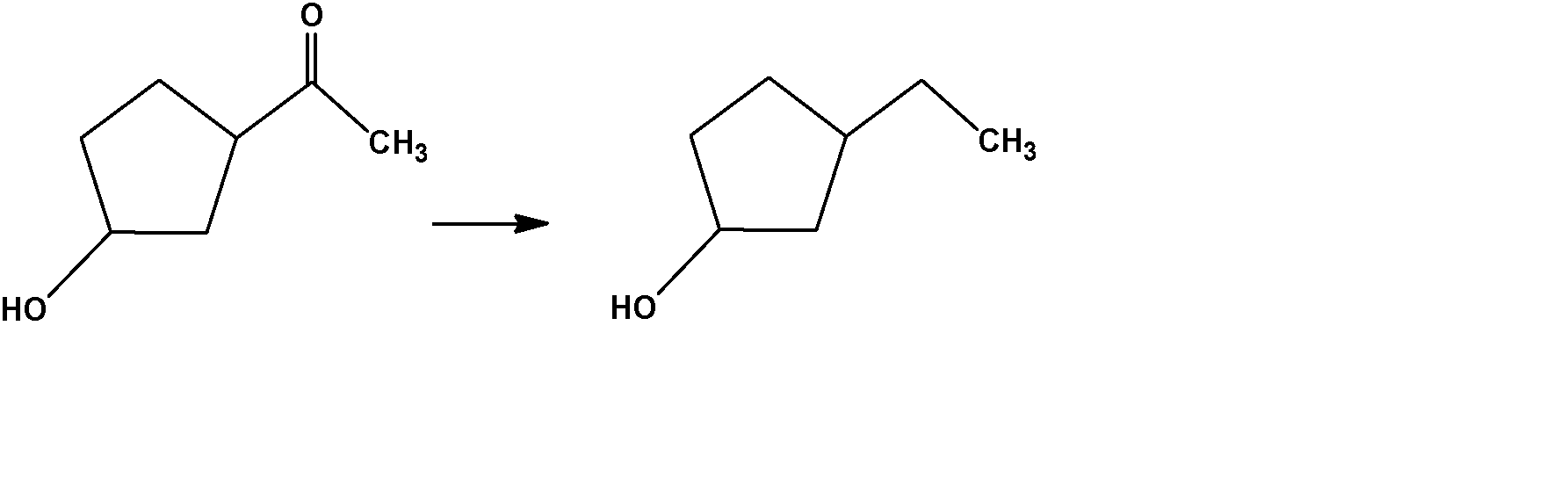
$A.\,\,Zn - Hg/HCl$
$B.\,\,{H_2}N - N{H_2},KOH/ethyleneglycol$
$C.\,\,Ni/{H_2}$
$D.\,\,NaB{H_4}$

Answer
550.8k+ views
Hint:Both Wolff-Kishner and Clemmensen reduction are used in the reaction. The option in which attack –on OH group of the ring leading to reduction of phenolic group is also not applicable. In both the carbonyl compounds gets reduced but the difference only comes with the acid and base sensitive reactants. Suppose if a reactant is acid sensitive the Wolff-Kishner is best whereas, in the case of base sensitive compounds the Clemmensen reduction is best.
Complete step-by-step answer:Wolff- Kishner reaction involves the reduction of aldehydes and ketones to alkanes. Condensation of the carbonyl compound with hydrazine forms the hydrazone, and treatment with base induces the reduction of the carbon coupled with oxidation of the hydrazine to gaseous nitrogen, to yield the corresponding alkane.
For the reduction of $C = O$ group to $ - C{H_2} - $ group Wolff-Kishner reagent $N{H_2}N{H_2}OH$ is preferred. Clemmensen reduction is a chemical reaction described as a reduction of ketones (or aldehydes) to alkanes using zinc amalgam and concentrated hydrochloric acid.
Wolff Kishner reagent is preferred over Clemmensen reaction to avid the reduction of phenolic $OH$ group. In this reaction is the formation of a hydrazone anion 1 by deprotonation of the terminal nitrogen takes place.
$Ni/{H_2}$ is used as a catalyst in the hydrogenation type of reactions. Similarly, $NaB{H_4}$Sodium borohydride, a representative borohydride reagent, behaves as an effective source of nucleophilic hydride in an aprotic polar solvent and is used for the reduction of alkyl halides.
Even in the case of $Zn - Hg/HCl$ the reduction reaction product will be chloro-derivative.
Therefore, the correct option is $N{H_2}N{H_2}OH$ which will give the aforesaid product. Therefore, the right option is an option (B).
Note:The Clemmensen Reduction is complementary to the Wolff-Kishner Reduction. These both are different because few reactants are acidic/base sensitive. The Clemmensen reduction is run under strongly basic conditions. Both $Zn - Hg/HCl$ (Clemmensen's reduction) and ${H_2}N - N{H_2},KOH/ethyleneglycol$ (Wolf Kishner reduction) can be used but in case of $Zn - Hg/HCl$ the product will not be alcohol but a chloro-derivative.
Complete step-by-step answer:Wolff- Kishner reaction involves the reduction of aldehydes and ketones to alkanes. Condensation of the carbonyl compound with hydrazine forms the hydrazone, and treatment with base induces the reduction of the carbon coupled with oxidation of the hydrazine to gaseous nitrogen, to yield the corresponding alkane.
For the reduction of $C = O$ group to $ - C{H_2} - $ group Wolff-Kishner reagent $N{H_2}N{H_2}OH$ is preferred. Clemmensen reduction is a chemical reaction described as a reduction of ketones (or aldehydes) to alkanes using zinc amalgam and concentrated hydrochloric acid.
Wolff Kishner reagent is preferred over Clemmensen reaction to avid the reduction of phenolic $OH$ group. In this reaction is the formation of a hydrazone anion 1 by deprotonation of the terminal nitrogen takes place.
$Ni/{H_2}$ is used as a catalyst in the hydrogenation type of reactions. Similarly, $NaB{H_4}$Sodium borohydride, a representative borohydride reagent, behaves as an effective source of nucleophilic hydride in an aprotic polar solvent and is used for the reduction of alkyl halides.
Even in the case of $Zn - Hg/HCl$ the reduction reaction product will be chloro-derivative.
Therefore, the correct option is $N{H_2}N{H_2}OH$ which will give the aforesaid product. Therefore, the right option is an option (B).
Note:The Clemmensen Reduction is complementary to the Wolff-Kishner Reduction. These both are different because few reactants are acidic/base sensitive. The Clemmensen reduction is run under strongly basic conditions. Both $Zn - Hg/HCl$ (Clemmensen's reduction) and ${H_2}N - N{H_2},KOH/ethyleneglycol$ (Wolf Kishner reduction) can be used but in case of $Zn - Hg/HCl$ the product will not be alcohol but a chloro-derivative.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




