
The amine which will not liberate nitrogen with nitrous acid is:
A) Ethylamine
B) Methylamine
C) Dimethylamine
D) 2-amino propane
Answer
558.9k+ views
Hint: Amines are known as derivatives of ammonia in which one or more of the hydrogen atoms of ammonia have been replaced by alkyl groups. There are three types of amines; primary, secondary and tertiary depending upon the number of alkyl groups attached to the nitrogen atom of ammonia. Each type of amine gives a different product on reaction with nitrous acid.
Complete step-by-step answer:
The chemical formula of ammonia is as follows:

Replacement of one hydrogen atom of ammonia by the alkyl group gives primary ammonia. The general formula of primary amine is as follows:
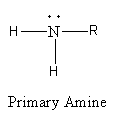
Replacement of two hydrogen atoms of ammonia by alkyl groups gives secondary ammonia. The general formula of secondary amine is as follows:
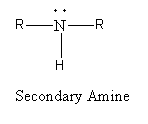
Replacement of three hydrogen atoms of ammonia by alkyl groups gives tertiary ammonia. The general formula of tertiary amine is as follows:
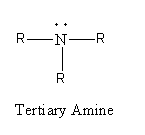
Now, we will see the reaction of each type of amine with nitrous acid. The chemical formula of nitrous acid is \[{\text{HN}}{{\text{O}}_{\text{2}}}\].
Primary amines react with nitrous acid and give alcohol, nitrogen gas and water as the products.
\[{\text{RN}}{{\text{H}}_{\text{2}}}{\text{ + HN}}{{\text{O}}_{\text{2}}} \to {\text{ROH + }}{{\text{N}}_{\text{2}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
Secondary amines react with nitrous acid and give yellow colour nitrosamine and water as the products.
\[{{\text{R}}_{\text{2}}}{\text{NH + HN}}{{\text{O}}_{\text{2}}} \to {{\text{R}}_{\text{2}}}{\text{N - N = O + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
Tertiary amines react with nitrous acid and give trialkyl ammonium ions as the products. \[{{\text{R}}_3}{\text{N + HN}}{{\text{O}}_{\text{2}}} \to {{\text{R}}_3}{\text{N}}{{\text{H}}^{\text{ + }}}\]
Thus, we can say that only primary amines react with nitrous acid liberated nitrogen gas. Now to determine which amine will not liberate nitrogen with nitrous acid we have to determine type amine.
The formula of ethyl amine is \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\]. As it is a primary amine, it will liberate nitrogen on reaction with nitrous acid. Hence, we can say that option (A) Ethylamine is an incorrect answer.
The formula of methyl amine is\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}\]. As it is a primary amine, it will liberate nitrogen on reaction with nitrous acid. Hence, we can say that option (B) Methylamine is an incorrect answer.
The formula of Dimethyl amine is\[{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{NH}}\]. As it is a secondary amine, it will not liberate nitrogen on reaction with nitrous acid. Hence, we can say that option (C) Dimethylamine is the correct answer.
The formula of 2-amino propane is
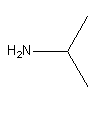
As it is a primary amine, it will liberate nitrogen on reaction with nitrous acid. Hence, we can say that option (B) Dimethylamine is an incorrect answer.
Hence the correct answer is option ‘C’.
Note: Primary, secondary and tertiary amines show different reactions with nitrous acid. Only primary amines liberate nitrogen gas on reaction with nitrous acid. So we can use nitrous acid reagent to distinguish primary, secondary and tertiary amines.
Complete step-by-step answer:
The chemical formula of ammonia is as follows:

Replacement of one hydrogen atom of ammonia by the alkyl group gives primary ammonia. The general formula of primary amine is as follows:

Replacement of two hydrogen atoms of ammonia by alkyl groups gives secondary ammonia. The general formula of secondary amine is as follows:
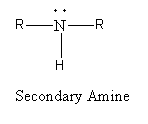
Replacement of three hydrogen atoms of ammonia by alkyl groups gives tertiary ammonia. The general formula of tertiary amine is as follows:

Now, we will see the reaction of each type of amine with nitrous acid. The chemical formula of nitrous acid is \[{\text{HN}}{{\text{O}}_{\text{2}}}\].
Primary amines react with nitrous acid and give alcohol, nitrogen gas and water as the products.
\[{\text{RN}}{{\text{H}}_{\text{2}}}{\text{ + HN}}{{\text{O}}_{\text{2}}} \to {\text{ROH + }}{{\text{N}}_{\text{2}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
Secondary amines react with nitrous acid and give yellow colour nitrosamine and water as the products.
\[{{\text{R}}_{\text{2}}}{\text{NH + HN}}{{\text{O}}_{\text{2}}} \to {{\text{R}}_{\text{2}}}{\text{N - N = O + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
Tertiary amines react with nitrous acid and give trialkyl ammonium ions as the products. \[{{\text{R}}_3}{\text{N + HN}}{{\text{O}}_{\text{2}}} \to {{\text{R}}_3}{\text{N}}{{\text{H}}^{\text{ + }}}\]
Thus, we can say that only primary amines react with nitrous acid liberated nitrogen gas. Now to determine which amine will not liberate nitrogen with nitrous acid we have to determine type amine.
The formula of ethyl amine is \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\]. As it is a primary amine, it will liberate nitrogen on reaction with nitrous acid. Hence, we can say that option (A) Ethylamine is an incorrect answer.
The formula of methyl amine is\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}\]. As it is a primary amine, it will liberate nitrogen on reaction with nitrous acid. Hence, we can say that option (B) Methylamine is an incorrect answer.
The formula of Dimethyl amine is\[{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{NH}}\]. As it is a secondary amine, it will not liberate nitrogen on reaction with nitrous acid. Hence, we can say that option (C) Dimethylamine is the correct answer.
The formula of 2-amino propane is

As it is a primary amine, it will liberate nitrogen on reaction with nitrous acid. Hence, we can say that option (B) Dimethylamine is an incorrect answer.
Hence the correct answer is option ‘C’.
Note: Primary, secondary and tertiary amines show different reactions with nitrous acid. Only primary amines liberate nitrogen gas on reaction with nitrous acid. So we can use nitrous acid reagent to distinguish primary, secondary and tertiary amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




