
The amine that reacts with $NaN{{O}_{2}}+HCl$ to give oily yellow liquid is:
[A] Ethyl amine.
[B] Dimethyl amine.
[C] Isopropyl amine.
[D] Secondary butyl amine.
Answer
592.2k+ views
Hint: In presence of sodium nitrite and hydrochloric acid, amines undergo diazotization. Primary amines form a mixture of products and secondary amines cannot complete the diazotization reaction and gives us an oily yellow liquid. Here, the correct answer is a secondary amine.
Complete answer:
The reagent given to us is sodium nitrite which is an inorganic compound and we use it in industrial chemistry for the formation of diazo compounds from amines which is used for the preparation of various diazo dyes.
A diazo group is an organic group which consists of two nitrogen atoms linked together in the terminal position. The nitrogen atoms are termed –azo groups therefore the name di-azo.
An amine will undergo diazotization when it reacts with sodium nitrite in presence of hydrochloric acid.
When we react with a primary amine with sodium nitrite we get an unstable salt of ethyl diazonium chloride. The salt is unstable thus decomposes into a cation and reacts with any nucleophile present in the solution and gives us a mixture of alcohol, alkene and alkyl halides none of which is an oily yellow liquid.
Primary amines are the amines having only one carbon attached to a nitrogen atom.
We can draw the structures of the given amines as –
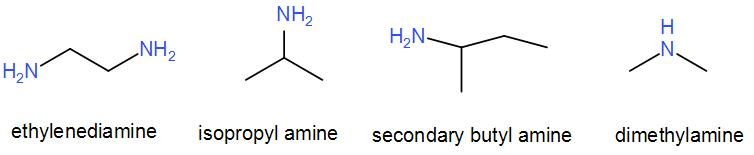
As we can see from the above diagram, among the given options, ethyl amine, secondary butyl amine and isopropyl amine are primary amines. Therefore, neither of them is the correct option.
All the primary amines will give a mixture of alcohol, alkyl halides and alkenes thus we are left with dimethyl amine so let's discuss its reaction with sodium nitrite and hydrochloric acid.
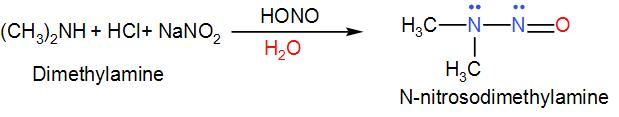
As we can see in the above reaction, when dimethyl amine undergoes diazotization, we get N-nitrosodimethylamine which is an oily yellow liquid.
So, the correct answer is “Option B”.
Note: Primary amines give us a diazonium salt which forms alcohols in addition to water but secondary amines have one hydrogen atom attached to them therefore, they cannot complete the diazotization reaction and give us a yellow oily nitrosamine product. Three degree amines have no hydrogen atoms attached to them thus they undergo simple acid- base reactions and give us soluble salts.
Complete answer:
The reagent given to us is sodium nitrite which is an inorganic compound and we use it in industrial chemistry for the formation of diazo compounds from amines which is used for the preparation of various diazo dyes.
A diazo group is an organic group which consists of two nitrogen atoms linked together in the terminal position. The nitrogen atoms are termed –azo groups therefore the name di-azo.
An amine will undergo diazotization when it reacts with sodium nitrite in presence of hydrochloric acid.
When we react with a primary amine with sodium nitrite we get an unstable salt of ethyl diazonium chloride. The salt is unstable thus decomposes into a cation and reacts with any nucleophile present in the solution and gives us a mixture of alcohol, alkene and alkyl halides none of which is an oily yellow liquid.
Primary amines are the amines having only one carbon attached to a nitrogen atom.
We can draw the structures of the given amines as –

As we can see from the above diagram, among the given options, ethyl amine, secondary butyl amine and isopropyl amine are primary amines. Therefore, neither of them is the correct option.
All the primary amines will give a mixture of alcohol, alkyl halides and alkenes thus we are left with dimethyl amine so let's discuss its reaction with sodium nitrite and hydrochloric acid.

As we can see in the above reaction, when dimethyl amine undergoes diazotization, we get N-nitrosodimethylamine which is an oily yellow liquid.
So, the correct answer is “Option B”.
Note: Primary amines give us a diazonium salt which forms alcohols in addition to water but secondary amines have one hydrogen atom attached to them therefore, they cannot complete the diazotization reaction and give us a yellow oily nitrosamine product. Three degree amines have no hydrogen atoms attached to them thus they undergo simple acid- base reactions and give us soluble salts.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




