
The acid in which O-O bonding is present is:
A.\[{H_2}{S_2}{O_3}\]
B.\[{H_2}{S_2}{O_6}\]
C.\[{H_2}{S_2}{O_8}\]
D.\[{H_2}{S_4}{O_6}\]
Answer
598.8k+ views
Hint: An O-O linkage is known as a peroxy linkage. The compounds which have an -O-O-H atomic group in place of an -O-H group are peroxy acids. They are also known as peracids.
Complete step by step answer:
Peroxy acids are the class of chemical compounds in which the -OH group is replaced by the atomic group -OOH. Peroxy acids usually are prepared by reaction of the oxy acid with hydrogen peroxide; small amounts of sulfuric or other strong acids often are used to accelerate the reaction of weak oxy acids. The peroxy acids are used primarily as oxidizing agents; they readily add oxygen to alkenes to give epoxides and are used to convert ketones to esters and amines to nitro compounds, amine oxides, or nitroso compounds.
The general formula of a peroxy acid is R-O-O-R’.
Carboxylic acids are better acids than peroxy acids because the carboxylate ion is stabilised by resonance between the two oxygens which is not the case in the conjugate base of peroxy acids.
Now, these oxyacids have special structures.
The structure of \[{H_2}{S_2}{O_3}\] is:
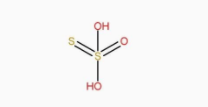
The structure of \[{H_2}{S_2}{O_6}\] is:

The structure of \[{H_2}{S_2}{O_8}\] is:
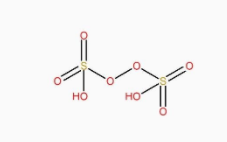
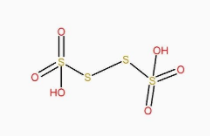
The structure of \[{H_2}{S_4}{O_6}\] is:
It can be clearly seen that \[{H_2}{S_2}{O_8}\] has peroxy linkage in it.
So, \[{H_2}{S_2}{O_8}\] is a peroxy acid.
Hence, the correct answer is (C)
Note: These oxyacids have special structures. So, one must remember these structures to answer this question correctly.
Complete step by step answer:
Peroxy acids are the class of chemical compounds in which the -OH group is replaced by the atomic group -OOH. Peroxy acids usually are prepared by reaction of the oxy acid with hydrogen peroxide; small amounts of sulfuric or other strong acids often are used to accelerate the reaction of weak oxy acids. The peroxy acids are used primarily as oxidizing agents; they readily add oxygen to alkenes to give epoxides and are used to convert ketones to esters and amines to nitro compounds, amine oxides, or nitroso compounds.
The general formula of a peroxy acid is R-O-O-R’.
Carboxylic acids are better acids than peroxy acids because the carboxylate ion is stabilised by resonance between the two oxygens which is not the case in the conjugate base of peroxy acids.
Now, these oxyacids have special structures.
The structure of \[{H_2}{S_2}{O_3}\] is:

The structure of \[{H_2}{S_2}{O_6}\] is:

The structure of \[{H_2}{S_2}{O_8}\] is:

The structure of \[{H_2}{S_4}{O_6}\] is:

It can be clearly seen that \[{H_2}{S_2}{O_8}\] has peroxy linkage in it.
So, \[{H_2}{S_2}{O_8}\] is a peroxy acid.
Hence, the correct answer is (C)
Note: These oxyacids have special structures. So, one must remember these structures to answer this question correctly.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




