
The absolute configuration of the compound is:


Answer
584.7k+ views
Hint: Suppose there are two test tubes, one containing (-) lactic acid and the other one has (+) enantiomers. How can you distinguish them? To generate a proper model to answer this dilemma, Rosanoff proposed that one compound be chosen as a standard and a configuration arbitrarily assigned to it.
Complete step-by-step answer:
- R- and S- notation use the CIP priority rules for the assignment of the absolute configuration around a stereocenter.
- First, assign priorities as described above to each bonded group surrounding the stereocenter (1, highest to 4, lowest) .
- Second, point the lowest priority (4) atom away from you. Follow the direction of the remaining 3 priorities from highest to lowest priority (lowest to highest number, 1<2<3).
- A counterclockwise direction is an S (sinister, Latin for left) configuration. A clockwise direction is an R (rectus, Latin for right) configuration.
R-S system RULES OF PRIORITY ORDER:
(1) According to the atomic number when only atoms are present and in a group the direct attached atom is considered with the atomic number.
(2) When two or more groups have similar first atoms, the priority is determined by considering the atomic number of the second atom.
(3) If the first atom of two same groups have the same substitutes of higher no will get more priority.
(4) More atomic numbers containing atoms and how many atoms are present of the higher atomic number containing elements.
Now, let us come to the compound given to us:
S→R
as H is in front
S→R
as H is in front we reverse the configuration obtained
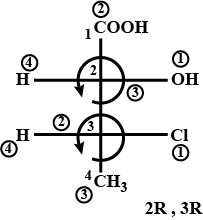
Note: Always find out the stereoisomers and then the chiral carbon. Then start using the CIP rule to assign R and S.
Complete step-by-step answer:
- R- and S- notation use the CIP priority rules for the assignment of the absolute configuration around a stereocenter.
- First, assign priorities as described above to each bonded group surrounding the stereocenter (1, highest to 4, lowest) .
- Second, point the lowest priority (4) atom away from you. Follow the direction of the remaining 3 priorities from highest to lowest priority (lowest to highest number, 1<2<3).
- A counterclockwise direction is an S (sinister, Latin for left) configuration. A clockwise direction is an R (rectus, Latin for right) configuration.
R-S system RULES OF PRIORITY ORDER:
(1) According to the atomic number when only atoms are present and in a group the direct attached atom is considered with the atomic number.
(2) When two or more groups have similar first atoms, the priority is determined by considering the atomic number of the second atom.
(3) If the first atom of two same groups have the same substitutes of higher no will get more priority.
(4) More atomic numbers containing atoms and how many atoms are present of the higher atomic number containing elements.
Now, let us come to the compound given to us:
S→R
as H is in front
S→R
as H is in front we reverse the configuration obtained

Note: Always find out the stereoisomers and then the chiral carbon. Then start using the CIP rule to assign R and S.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




