
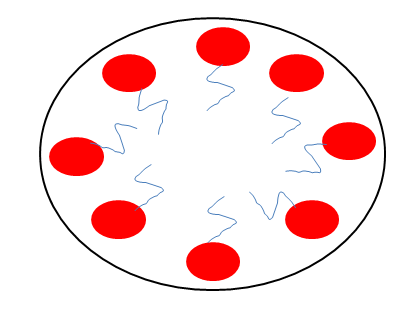
Surfactant molecules can cluster together as micelles, to be in colloid size. Micelles from only above critical micelle concentration (CMC) and above a certain temperature called kraft temperature. $\Delta H$ of micelle formation can be positive or negative. Which is false about micelle formation?
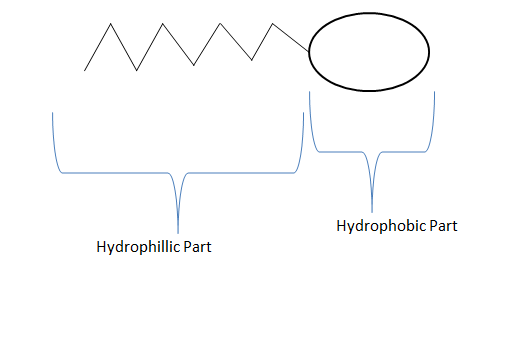
(A). The hydrophobic part lies towards the interior of micelle.
(B). The hydrophilic part lies towards the surface of the micelle.
(C). $\Delta S$ of micelle formation is positive.
(D). $\Delta S$ of micelle formation is negative
Answer
507k+ views
Hint: In general a micelle is an agreement of several surfactant molecules dispersed in a medium like liquid and leads to the formation of the colloidal suspension. According to I.U.P.A.C ( international union of pure and applied chemistry ) micelles are the very minute particles which are capable of forming an equilibrium with the ions or molecules in solution from which they are synthesised.
Complete answer:
Krafft temperature is the temperature required for the solubility of the surfactants to be equal to critical micelle concentration (CMC).
The increase in the length of the chain of a micelle, usually the hydrocarbon chain ( hydrophilic part ) will increase the required krafft temperature as well.
The most common example of micelle is the action of soap or the detergents.
The correct answer is option (C). $\Delta S$ of micelle formation is positive.
Note:
The critical micelle concentration (CMC) having surfactants can act as emulsifiers which helps in the desolation of the un- soluble particles in the liquid medium. Micelles are responsible for the absorption of various fat soluble vitamins, lipids in the human body. Micelles can also be used as an active drug delivery or drug target substrate.
Complete answer:
Krafft temperature is the temperature required for the solubility of the surfactants to be equal to critical micelle concentration (CMC).
The increase in the length of the chain of a micelle, usually the hydrocarbon chain ( hydrophilic part ) will increase the required krafft temperature as well.
The most common example of micelle is the action of soap or the detergents.
The correct answer is option (C). $\Delta S$ of micelle formation is positive.


Note:
The critical micelle concentration (CMC) having surfactants can act as emulsifiers which helps in the desolation of the un- soluble particles in the liquid medium. Micelles are responsible for the absorption of various fat soluble vitamins, lipids in the human body. Micelles can also be used as an active drug delivery or drug target substrate.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




