
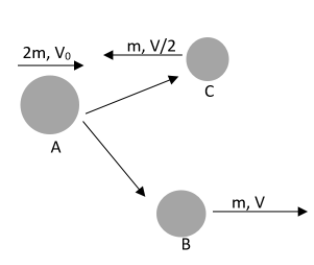
Suppose that a nucleus A, having a specific de - Broglie wavelength ${{\lambda }_{A}}$gets divided into two nuclei B and C of equal mass because of the spontaneous fission. At this situation, B flies in the identical direction as that of A. But C flies in the direction opposite to this with a velocity equivalent to half of that of B. calculate the de Broglie wavelengths ${{\lambda }_{B}}$ and ${{\lambda }_{C}}$ of B and C?
$\begin{align}
& A.2{{\lambda }_{A}},{{\lambda }_{A}} \\
& B.{{\lambda }_{A}},2{{\lambda }_{A}} \\
& C.{{\lambda }_{A}},\dfrac{{{\lambda }_{A}}}{2} \\
& D.\dfrac{{{\lambda }_{A}}}{2},{{\lambda }_{A}} \\
\end{align}$
Answer
571.5k+ views
Hint: Apply the conservation of momentum at first as per the mentioning in the question. Then find out the velocity of A. Using these values, find out the de Broglie wavelength which will be the ratio of the Planck’s constant to the momentum of the body. This will help you in answering this question.
Complete step by step answer:
It has been mentioned in the question that the mass of the nuclei, B and C are equal. Therefore let us assume that the mass of both the bodies is $m$. And also it has been mentioned that the velocity of C is half of that of B and they are moving in opposite directions. Therefore we can write that,
${{V}_{C}}=-\dfrac{{{V}_{B}}}{2}$
And the velocity of B is in the same direction of A also. We can apply the momentum conservation here. It can be written as,
$2m{{V}_{A}}=mV-m\dfrac{V}{2}$
Simplifying this equation will give the value of the velocity of the nucleus A.
That is,
$V=4{{V}_{A}}$
The momentum corresponding to each of the nuclei can be written as,
$\begin{align}
& {{P}_{A}}=2m{{V}_{0}} \\
& {{P}_{B}}=4m{{V}_{0}} \\
& {{P}_{C}}=2m{{V}_{0}} \\
\end{align}$
Therefore the de Broglie wavelength can be written as,
$\lambda =\dfrac{h}{P}$
Where $h$ be the Planck’s constant.
Hence the de Broglie wavelength of each of the nuclei will be,
$\begin{align}
& {{\lambda }_{A}}=\dfrac{h}{2m{{V}_{0}}} \\
& {{\lambda }_{B}}=\dfrac{h}{4m{{V}_{0}}} \\
& {{\lambda }_{C}}=\dfrac{h}{2m{{V}_{0}}} \\
\end{align}$
Therefore the relation between wavelengths of B and C with respect to A will be,
$\begin{align}
& {{\lambda }_{B}}=\dfrac{{{\lambda }_{A}}}{2} \\
& {{\lambda }_{C}}={{\lambda }_{A}} \\
\end{align}$
The answer is given as option D.
Note:
According to the dual nature of the wave, the De Broglie wavelength is a wavelength which can be related to all the bodies in quantum mechanics which represents the probability density of detecting the particle at a specific position of the configuration space.
Complete step by step answer:
It has been mentioned in the question that the mass of the nuclei, B and C are equal. Therefore let us assume that the mass of both the bodies is $m$. And also it has been mentioned that the velocity of C is half of that of B and they are moving in opposite directions. Therefore we can write that,
${{V}_{C}}=-\dfrac{{{V}_{B}}}{2}$
And the velocity of B is in the same direction of A also. We can apply the momentum conservation here. It can be written as,
$2m{{V}_{A}}=mV-m\dfrac{V}{2}$
Simplifying this equation will give the value of the velocity of the nucleus A.
That is,
$V=4{{V}_{A}}$
The momentum corresponding to each of the nuclei can be written as,
$\begin{align}
& {{P}_{A}}=2m{{V}_{0}} \\
& {{P}_{B}}=4m{{V}_{0}} \\
& {{P}_{C}}=2m{{V}_{0}} \\
\end{align}$
Therefore the de Broglie wavelength can be written as,
$\lambda =\dfrac{h}{P}$
Where $h$ be the Planck’s constant.
Hence the de Broglie wavelength of each of the nuclei will be,
$\begin{align}
& {{\lambda }_{A}}=\dfrac{h}{2m{{V}_{0}}} \\
& {{\lambda }_{B}}=\dfrac{h}{4m{{V}_{0}}} \\
& {{\lambda }_{C}}=\dfrac{h}{2m{{V}_{0}}} \\
\end{align}$
Therefore the relation between wavelengths of B and C with respect to A will be,
$\begin{align}
& {{\lambda }_{B}}=\dfrac{{{\lambda }_{A}}}{2} \\
& {{\lambda }_{C}}={{\lambda }_{A}} \\
\end{align}$
The answer is given as option D.

Note:
According to the dual nature of the wave, the De Broglie wavelength is a wavelength which can be related to all the bodies in quantum mechanics which represents the probability density of detecting the particle at a specific position of the configuration space.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




