
Sulphur is used in vulcanization of rubbers.
a. True
b. False
Answer
561.3k+ views
Hint: Vulcanization is the term used for the process of hardening natural rubber and stiffening it, in order to improve its properties and make it more durable. This mainly happens by the crosslinking of its monomer units which increase hardness.
Colloidal particles possess charge in them.
Complete step by step answer:
Vulcanization of rubber is the process of heating rubber in presence of Sulphur. The crude (raw) rubber is heated with 3 percent Sulphur at about 1200 degree Celsius for 2-3 hours. Natural rubber is not very strong in nature and therefore, it softens on heating. An additive can be added at this step.
- As a result of this, the chains of rubber and sulphur cross-link at reactive sites of double bonds with each other to form a strong and stable polymer.
The reaction taking place is as shown below,
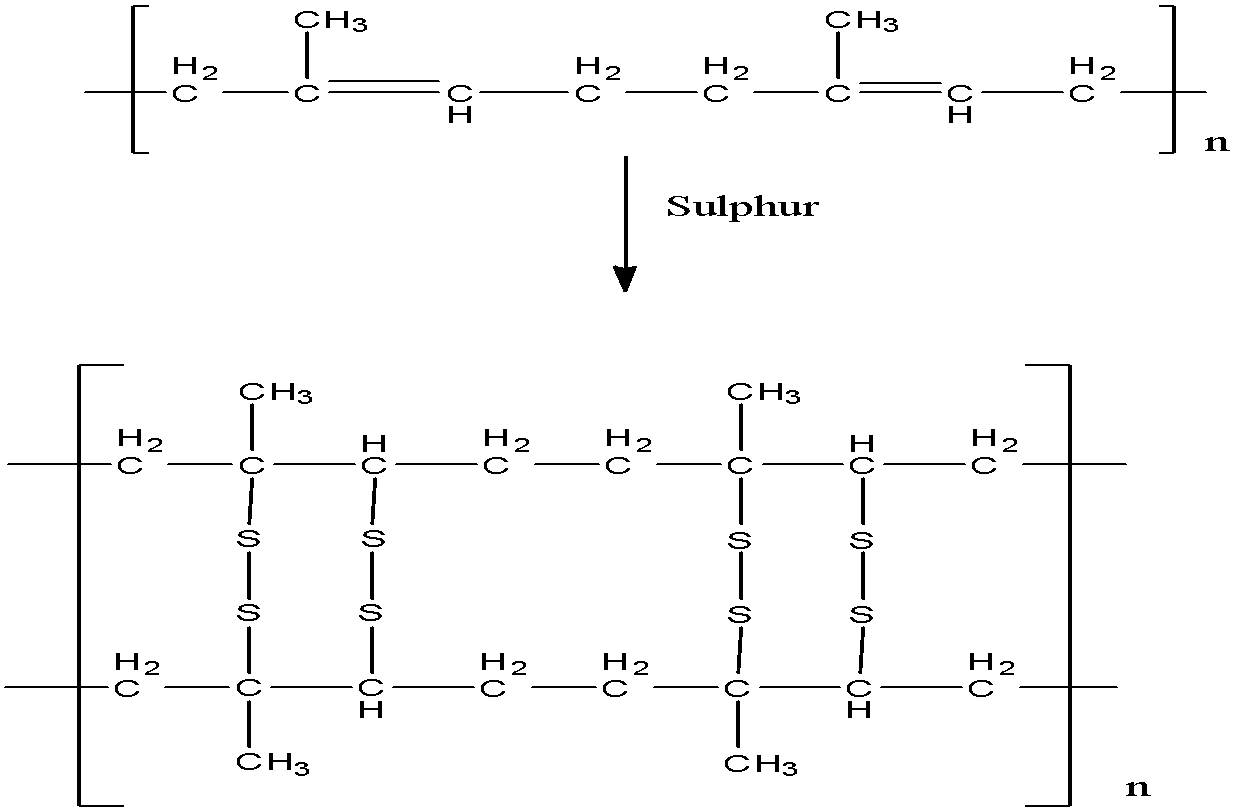
The temperature should be taken care of. At higher temperature (above 335 K) rubber becomes soft, whereas at lower temperature, rubber becomes brittle and it also shows high water absorption capacity. The correct option is option “A” .
Additional Information :Natural rubber is also known as cis-1,4-polyisoprene as it is made by polymerization of isoprene.
Note: Rubbers are categorized as natural and synthetic. Natural rubbers are the elastomers that are obtained naturally. It is composed of isoprene monomers whereas synthetic rubbers are derived from petroleum, natural gas and polymerization of butadiene.
Colloidal particles possess charge in them.
Complete step by step answer:
Vulcanization of rubber is the process of heating rubber in presence of Sulphur. The crude (raw) rubber is heated with 3 percent Sulphur at about 1200 degree Celsius for 2-3 hours. Natural rubber is not very strong in nature and therefore, it softens on heating. An additive can be added at this step.
- As a result of this, the chains of rubber and sulphur cross-link at reactive sites of double bonds with each other to form a strong and stable polymer.
The reaction taking place is as shown below,

The temperature should be taken care of. At higher temperature (above 335 K) rubber becomes soft, whereas at lower temperature, rubber becomes brittle and it also shows high water absorption capacity. The correct option is option “A” .
Additional Information :Natural rubber is also known as cis-1,4-polyisoprene as it is made by polymerization of isoprene.
Note: Rubbers are categorized as natural and synthetic. Natural rubbers are the elastomers that are obtained naturally. It is composed of isoprene monomers whereas synthetic rubbers are derived from petroleum, natural gas and polymerization of butadiene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




