
Structure of $Xe{F_6}$ is octahedron with $s{p^3}{d^3}$ hybridization of $Xe$ .
A.True
B.False
Answer
561.6k+ views
Hint:We can find out the hybridization of an atom in a compound, utilizing a steric number. We can compute the steric number by summarizing the number of atoms attached to the center molecule and the solitary sets of electrons on the central metal particles. Tell us that on the off chance that the steric number is 4, at that point we state the molecule is in $s{p^3}$ hybridization, on the off chance that the steric number is 3, at that point, it is $s{p^2}$ hybridization, if the steric number is 2, at that point it is $sp$ hybridization.
Complete answer:
We can draw the structure of $Xe{F_6}$ as,
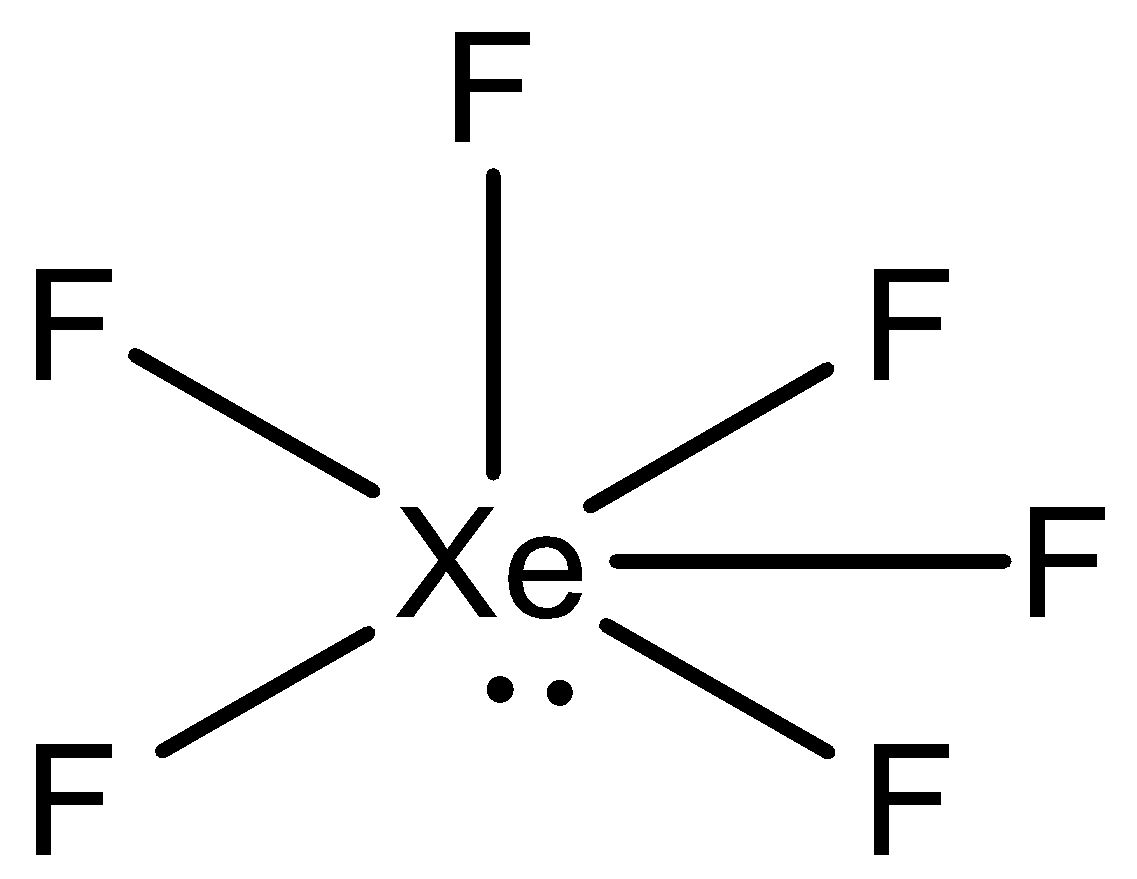
During the arrangement of $Xe{F_6}$ , xenon has eight electrons in its valence shell and it creates six bonds with the fluorine atoms. Further, the atoms will have one lone pair of electrons and six bond pairs. Presently on the off chance that we take the steric number, at that point, it will be seven. This can be deciphered as $s{p^3}{d^3}$ hybridization.
After hybridization, $Xe{F_6}$ molecular geometry would be distorted octahedral or square bipyramidal. What takes place here is that the atoms of fluorine atoms are located in the apexes of the octahedron while the lone pairs travel in the space to evade or decrease the repulsion.
Therefore, the option (B) is correct.
Note:
We have to remember that, $Xe{F_6}$ contains seven pairs of electrons. In that the number of bond pairs is six and the number of lone pairs is one. The number of electrons in the outermost shell of xenon is eight. When the fluorides of xenon have made electrons in the outermost shell of xenon get unpaired and are moved to empty $5d$ orbital.
Complete answer:
We can draw the structure of $Xe{F_6}$ as,

During the arrangement of $Xe{F_6}$ , xenon has eight electrons in its valence shell and it creates six bonds with the fluorine atoms. Further, the atoms will have one lone pair of electrons and six bond pairs. Presently on the off chance that we take the steric number, at that point, it will be seven. This can be deciphered as $s{p^3}{d^3}$ hybridization.
After hybridization, $Xe{F_6}$ molecular geometry would be distorted octahedral or square bipyramidal. What takes place here is that the atoms of fluorine atoms are located in the apexes of the octahedron while the lone pairs travel in the space to evade or decrease the repulsion.
Therefore, the option (B) is correct.
Note:
We have to remember that, $Xe{F_6}$ contains seven pairs of electrons. In that the number of bond pairs is six and the number of lone pairs is one. The number of electrons in the outermost shell of xenon is eight. When the fluorides of xenon have made electrons in the outermost shell of xenon get unpaired and are moved to empty $5d$ orbital.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




