
What is the structure of glycine at pH 5?
Answer
493.5k+ views
Hint: In the statistical mean, the isoelectric point is the pH at which a molecule has no net electrical charge or is electrically neutral. pH is the conventional abbreviation for the isoelectric point (I). pI, on the other hand, is also utilised. This article employs the abbreviation pI for the sake of brevity. The pH of the surrounding environment affects the net charge on the molecule, which might become more positively or negatively charged due to the addition or loss of protons (${{H}^{+}}$).
Complete answer:
The amino acid glycine has a single hydrogen atom in its side chain. It has the chemical formula \[N{{H}_{2}}C{{H}_{2}}COOH\] and is the simplest stable amino acid (carbamic acid is unstable). One of the proteinogenic amino acids is glycine. It is encoded by all codons that begin with the letter GG (GGU, GGC, GGA, GGG). Because of its compact nature, glycine is essential for the development of alpha-helices in secondary protein structure. It is also the most prevalent amino acid in collagen triple-helices for the same reason. Glycine is also an inhibitory neurotransmitter, and blocking its release in the spinal cord can result in spastic paralysis from uncontrolled muscular contraction.
It is primarily notable for its acid–base characteristics. Glycine is amphoteric in aqueous solution: at low pH, it may be protonated with a \[p{{K}_{a}}\] of approximately 2.4, while at high pH, it loses a proton with a \[p{{K}_{a}}\] of about 9.6. (precise values of \[p{{K}_{a}}\] depend on temperature and ionic strength).
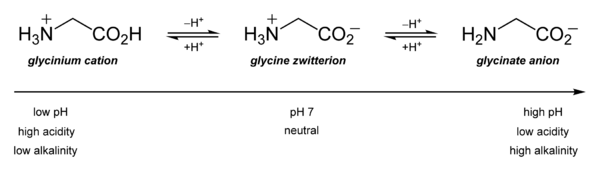
\[pI=\dfrac{p{{K}_{a1}}+p{{K}_{a2}}}{2}=\dfrac{2.34+9.60}{2}=\dfrac{11.94}{2}=5.97\]
Glycine has a net positive charge below pH 5.97, but it has a net negative charge above pH 5.97.
Hence it is isoelectronic at pH 5.
Note:
Although glycine can be extracted from hydrolyzed protein, it is not employed in industrial manufacturing since chemical synthesis is more convenient. The amination of chloroacetic acid with ammonia, which produces glycine and ammonium chloride, and the Strecker amino acid synthesis, which is used in the United States and Japan, are the two primary methods. This method produces around 15 thousand tonnes per year.
Complete answer:
The amino acid glycine has a single hydrogen atom in its side chain. It has the chemical formula \[N{{H}_{2}}C{{H}_{2}}COOH\] and is the simplest stable amino acid (carbamic acid is unstable). One of the proteinogenic amino acids is glycine. It is encoded by all codons that begin with the letter GG (GGU, GGC, GGA, GGG). Because of its compact nature, glycine is essential for the development of alpha-helices in secondary protein structure. It is also the most prevalent amino acid in collagen triple-helices for the same reason. Glycine is also an inhibitory neurotransmitter, and blocking its release in the spinal cord can result in spastic paralysis from uncontrolled muscular contraction.
It is primarily notable for its acid–base characteristics. Glycine is amphoteric in aqueous solution: at low pH, it may be protonated with a \[p{{K}_{a}}\] of approximately 2.4, while at high pH, it loses a proton with a \[p{{K}_{a}}\] of about 9.6. (precise values of \[p{{K}_{a}}\] depend on temperature and ionic strength).

\[pI=\dfrac{p{{K}_{a1}}+p{{K}_{a2}}}{2}=\dfrac{2.34+9.60}{2}=\dfrac{11.94}{2}=5.97\]
Glycine has a net positive charge below pH 5.97, but it has a net negative charge above pH 5.97.
Hence it is isoelectronic at pH 5.
Note:
Although glycine can be extracted from hydrolyzed protein, it is not employed in industrial manufacturing since chemical synthesis is more convenient. The amination of chloroacetic acid with ammonia, which produces glycine and ammonium chloride, and the Strecker amino acid synthesis, which is used in the United States and Japan, are the two primary methods. This method produces around 15 thousand tonnes per year.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




