
What is the structure of barium bromide in an aqueous solution?
Answer
478.2k+ views
Hint: Barium bromide is an ionic compound in which barium loses electrons and bromine accepts electrons. Thus when it is added to water then the ionic compound will first break into its constituent ions. Then the water molecule will surround the cation of the barium bromide which is the structure of barium bromide in an aqueous solution.
Complete Answer:
When barium loses a pair of electrons and chlorine accepts the pair of electrons then barium bromide is formed. Thus it is an ionic compound which forms by transfer of electrons from barium to bromine atom. Thus when we dissolve barium bromide into water then its ions gets separated and it may be represented as:
\[BaB{r_2}{\text{ }}\xrightarrow{{{H_2}O}}{\text{ }}B{a^{2 + }}{\text{ }} + {\text{ }}2B{r^ - }\]
Now we know that in water molecules oxygen has lone pairs of electrons. Since the barium bromide breaks into cation and anion respectively, the cation is barium ion \[\left( {B{a^{2 + }}} \right)\]. Now this cation will attract water molecules towards it and form a complex. The number of water molecules which will surround the barium cation is six. Therefore the structure of the compound will be as:
\[ \Rightarrow {\text{ }}{\left[ {Ba{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}\]
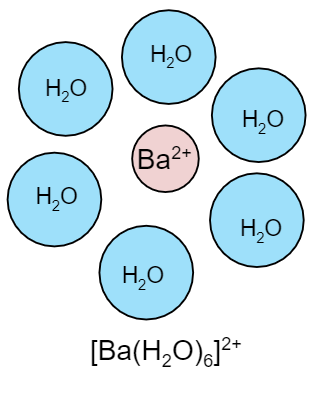
The above structure is hydrolyzed since it gets hydrolyzed by water molecules. There is the involvement of hydration energy while water molecules hydrolyze the barium cation.
Note:
It must be noted that for a compound to be hydrated, its hydration energy must be greater than its lattice energy. Thus for barium bromide, the hydration energy is greater than its lattice energy. When barium bromide gets dissociated into its constituent ions, dissociation energy plays an important factor.
Complete Answer:
When barium loses a pair of electrons and chlorine accepts the pair of electrons then barium bromide is formed. Thus it is an ionic compound which forms by transfer of electrons from barium to bromine atom. Thus when we dissolve barium bromide into water then its ions gets separated and it may be represented as:
\[BaB{r_2}{\text{ }}\xrightarrow{{{H_2}O}}{\text{ }}B{a^{2 + }}{\text{ }} + {\text{ }}2B{r^ - }\]
Now we know that in water molecules oxygen has lone pairs of electrons. Since the barium bromide breaks into cation and anion respectively, the cation is barium ion \[\left( {B{a^{2 + }}} \right)\]. Now this cation will attract water molecules towards it and form a complex. The number of water molecules which will surround the barium cation is six. Therefore the structure of the compound will be as:
\[ \Rightarrow {\text{ }}{\left[ {Ba{{\left( {{H_2}O} \right)}_6}} \right]^{2 + }}\]

The above structure is hydrolyzed since it gets hydrolyzed by water molecules. There is the involvement of hydration energy while water molecules hydrolyze the barium cation.
Note:
It must be noted that for a compound to be hydrated, its hydration energy must be greater than its lattice energy. Thus for barium bromide, the hydration energy is greater than its lattice energy. When barium bromide gets dissociated into its constituent ions, dissociation energy plays an important factor.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




