
How many structural isomeric primary amines are possible for the formula ${{C}_{4}}{{H}_{11}}N$?
(A) 2
(B) 3
(C) 4
(D) 5
Answer
576k+ views
Hint: In primary amines, only one carbon atom is directly attached to a nitrogen atom. Structural isomers have the same chemical formula and the same functional group. So make compounds that vary in the structure or the arrangement of carbon atoms without changing the bond between carbon and nitrogen atoms.
Complete step-by-step answer:
The word “isomer” is derived from the Greek words "isos" and "mers". "Isos" means equal and "mers" means parts, so "isomers" means equal parts. Isomerism is the phenomenon in which two or more compounds have the same chemical formula but differ in chemical structures. Chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecule are called isomers i.e. they exhibit isomerism. Isomerism is of two types namely, Structural isomerism and stereoisomerism. In structural isomerism the functional groups and the atoms in the molecules of these isomers are bonded in different ways. Structural isomers have different IUPAC names although their chemical formulae are the same.
The types of structural isomerism are:
- Chain
- Positional
- Functional
- Metamerism
- Tautomerism
- Ring - chain
We will now draw the structure of possible isomers of primary amine with formula ${{C}_{4}}{{H}_{11}}N$.
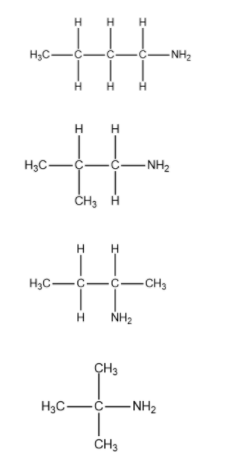
From the above explanation we can conclude that the total number of structural isomers for primary amine with formula ${{C}_{4}}{{H}_{11}}N$ is 4.
Therefore, the correct answer is option (C).
Note: The above structures are structural isomers of primary amine only. However, the total isomers include secondary and tertiary amines as well. Hence the total number of isomers for the amine with formula ${{C}_{4}}{{H}_{11}}N$ is 9.
Complete step-by-step answer:
The word “isomer” is derived from the Greek words "isos" and "mers". "Isos" means equal and "mers" means parts, so "isomers" means equal parts. Isomerism is the phenomenon in which two or more compounds have the same chemical formula but differ in chemical structures. Chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecule are called isomers i.e. they exhibit isomerism. Isomerism is of two types namely, Structural isomerism and stereoisomerism. In structural isomerism the functional groups and the atoms in the molecules of these isomers are bonded in different ways. Structural isomers have different IUPAC names although their chemical formulae are the same.
The types of structural isomerism are:
- Chain
- Positional
- Functional
- Metamerism
- Tautomerism
- Ring - chain
We will now draw the structure of possible isomers of primary amine with formula ${{C}_{4}}{{H}_{11}}N$.

From the above explanation we can conclude that the total number of structural isomers for primary amine with formula ${{C}_{4}}{{H}_{11}}N$ is 4.
Therefore, the correct answer is option (C).
Note: The above structures are structural isomers of primary amine only. However, the total isomers include secondary and tertiary amines as well. Hence the total number of isomers for the amine with formula ${{C}_{4}}{{H}_{11}}N$ is 9.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




