
What is the structural formula of kerosene?
Answer
523.2k+ views
Hint: Kerosene is a mixture of hydrocarbons consisting of $10$different types of hydrocarbons, each containing $10$to$16$different carbon atoms per molecule. The main constituents of kerosene are- saturated straight-chain and branched-chain paraffin, as well as ring-shaped cycloparaffins (also known as naphthenes). Although kerosene is less volatile than gasoline, it has a flash point (temperature at which it will generate flammable vapor near its surface) is ${38^ \circ }C$ or higher.
Complete answer: As we know, kerosene consists of $10$different types of hydrocarbons, the chemical composition depends on its source. Kerosene is a petroleum distillate which includes fractions with boiling points between ${150^ \circ }C$to${300^ \circ }C$.
Therefore, the structural formula of kerosene is${C_{12}}{H_{26}} - {C_{15}}{H_{32}}$.
We can also write the structure of kerosene as:
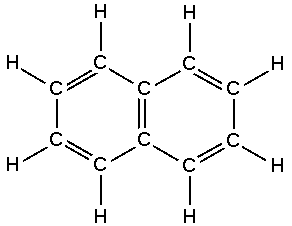
Fig: Structure of kerosene.
Note:
Like diesel, kerosene is also a straight-run fraction of petroleum and it is used as jet fuel as well as for domestic purposes. Kerosene is a low viscosity, clear liquid that is formed from the hydrocarbons obtained from fractional distribution of petroleum and has a density of $0.78 - 0.81gc{m^{ - 3}}$. It is mostly a mixture of hydrocarbons ranging from ${C_{10}}$to ${C_{16}}$ and must be free of aromatic and saturated hydrocarbons, as well as sulfur compounds. Typically, kerosene consists of $n - $alkanes, alkyl benzenes and naphthalenes.
Complete answer: As we know, kerosene consists of $10$different types of hydrocarbons, the chemical composition depends on its source. Kerosene is a petroleum distillate which includes fractions with boiling points between ${150^ \circ }C$to${300^ \circ }C$.
Therefore, the structural formula of kerosene is${C_{12}}{H_{26}} - {C_{15}}{H_{32}}$.
We can also write the structure of kerosene as:

Fig: Structure of kerosene.
Note:
Like diesel, kerosene is also a straight-run fraction of petroleum and it is used as jet fuel as well as for domestic purposes. Kerosene is a low viscosity, clear liquid that is formed from the hydrocarbons obtained from fractional distribution of petroleum and has a density of $0.78 - 0.81gc{m^{ - 3}}$. It is mostly a mixture of hydrocarbons ranging from ${C_{10}}$to ${C_{16}}$ and must be free of aromatic and saturated hydrocarbons, as well as sulfur compounds. Typically, kerosene consists of $n - $alkanes, alkyl benzenes and naphthalenes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




