
Structural formula $ - CON{H_2}$ is representing which of the following?
A) Amines.
B) Amide.
C) Alkyls.
D) Arenes.
Answer
569.7k+ views
Hint: We discuss the functional group.
Functional group:
We must remember that an atom or a group of atoms which decide the typical property of the organic compounds in spite of the size of molecule members of the similar functional group experiencing the same chemical reaction is called a functional group.
Complete answer:
Let us observe functional groups in detail.
A functional group might be an explicit group of particles or bonds with a compound that is at risk for the trademark synthetic responses of that compound and the atoms with the same useful gathering go through comparative response. Utilitarian gathering assumes a significant function in the terminology of a natural compound.
The particles in the functional group are connected together and to the compound through covalent bonds. An alpha carbon is the main carbon iota that appends with the utilitarian gathering and the subsequent carbon is called beta carbon. Contingent upon the connected carbon practical gatherings are referenced as essential, auxiliary and tertiary carbon particles.
Now, we observe about an amide functional group.
An amide functional group have nitrogen atom bonded to a carbonyl carbon atom
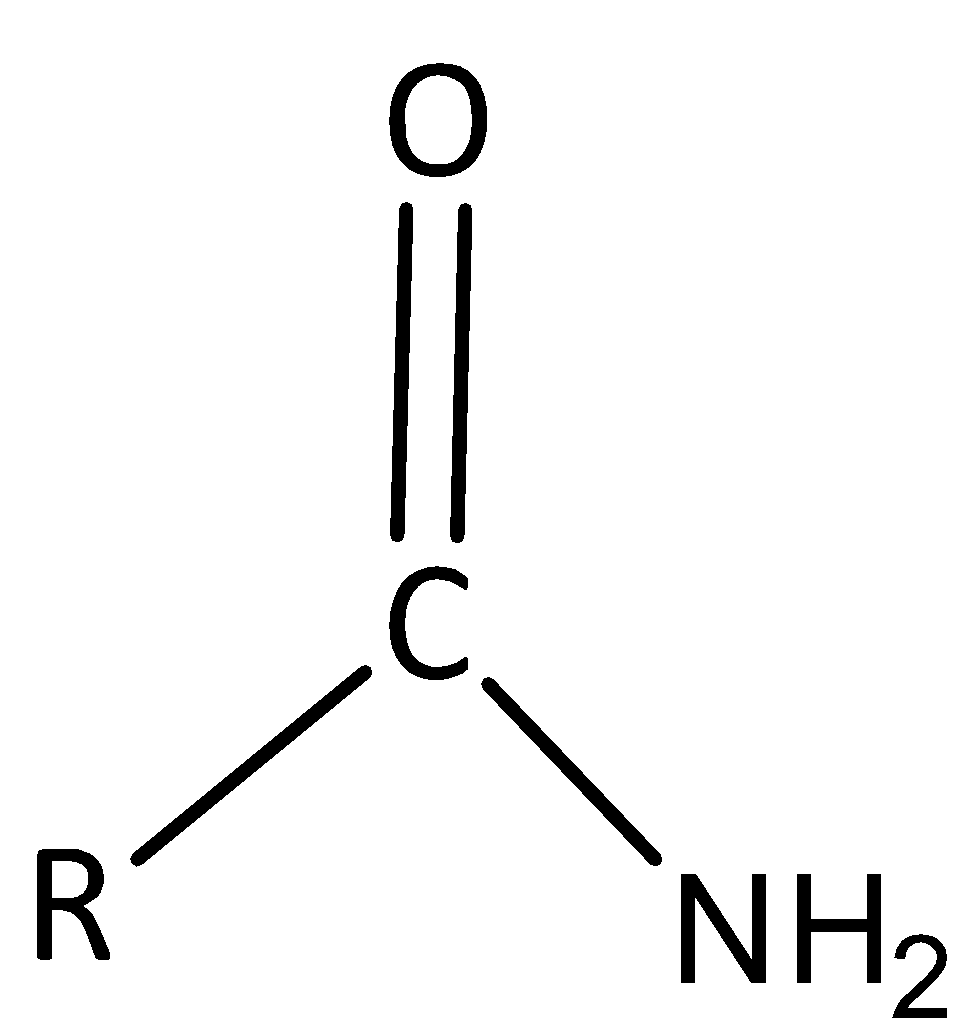
Thus option B is correct.
Basic structure \[ - CON{H_2}\] is symbolized to amide. Consequently, acetamide is symbolize as \[C{H_3}CON{H_2}\] while structural formula of Benzamide is \[{C_6}{H_5}CON{H_2}\].
The structural formula of amine is \[ - N{H_2}\].Thus option A is incorrect.
The structural formula of alkyl is \[ - R\]. Thus option C is incorrect and the Basic equation \[ - Ar\] is arenes. Thus option D is incorrect.
Therefore, the option B is correct.
Note: We have to know that a natural compound may contain more than one functional group. To distinguish the functional group one must be familiar with their formula.
Example: We know that the structural formula for hydroxyl functional group is $ - OH$ and the functional group for the acidic group is $ - COOH$.
Functional group:
We must remember that an atom or a group of atoms which decide the typical property of the organic compounds in spite of the size of molecule members of the similar functional group experiencing the same chemical reaction is called a functional group.
Complete answer:
Let us observe functional groups in detail.
A functional group might be an explicit group of particles or bonds with a compound that is at risk for the trademark synthetic responses of that compound and the atoms with the same useful gathering go through comparative response. Utilitarian gathering assumes a significant function in the terminology of a natural compound.
The particles in the functional group are connected together and to the compound through covalent bonds. An alpha carbon is the main carbon iota that appends with the utilitarian gathering and the subsequent carbon is called beta carbon. Contingent upon the connected carbon practical gatherings are referenced as essential, auxiliary and tertiary carbon particles.
Now, we observe about an amide functional group.
An amide functional group have nitrogen atom bonded to a carbonyl carbon atom

Thus option B is correct.
Basic structure \[ - CON{H_2}\] is symbolized to amide. Consequently, acetamide is symbolize as \[C{H_3}CON{H_2}\] while structural formula of Benzamide is \[{C_6}{H_5}CON{H_2}\].
The structural formula of amine is \[ - N{H_2}\].Thus option A is incorrect.
The structural formula of alkyl is \[ - R\]. Thus option C is incorrect and the Basic equation \[ - Ar\] is arenes. Thus option D is incorrect.
Therefore, the option B is correct.
Note: We have to know that a natural compound may contain more than one functional group. To distinguish the functional group one must be familiar with their formula.
Example: We know that the structural formula for hydroxyl functional group is $ - OH$ and the functional group for the acidic group is $ - COOH$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




