
Statement: The reagents \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] and excess of chlorine are required to prepare 1,1,2,2-tetrachloropropane from 1,2-dichloropropane.
If the given statement is true enter 1 if false enter 0.
Answer
588.9k+ views
Hint: Alkynes are prepared by dehydrohalogenation of dihalogen derivatives of alkanes in presence of a strong base. Alkynes on halogenation gives tetra halogen derivatives.
Complete step by step answer:
You can classify halogen derivatives of alkanes into mono halogen derivatives, di halogen derivatives, tri halogen derivatives, tetra halogen derivatives and poly halogen derivatives. mono halogen derivatives, di halogen derivatives, tri halogen derivatives, tetra halogen derivatives and poly halogen derivatives of alkanes contain one, two, three, four and several halogen atoms. Halogen can be either fluorine or chlorine or bromine or iodine.
\[{\text{NaN}}{{\text{H}}_{\text{2}}}\] is soda amide. It is a strong base. It is used for the dehydrohalogenation of haloalkanes. During dehydrohalogenation, hydrogen halide molecules are eliminated and carbon-carbon multiple bonds are obtained. Mono halogen derivative of alkane loses one molecule of hydrogen halide and forms an alkene. Dihalogen derivative of alkane loses two molecules of hydrogen halide and forms an alkyne.
1,2-dichloropropane is the dichloro derivative of alkane. When 1,2-dichloropropane is treated with \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] , it loses two molecules of hydrogen chloride to form propyne (an alkyne).
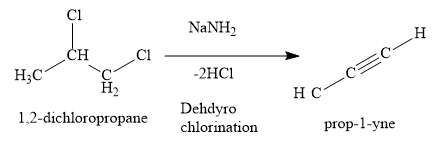
When propyne is treated with excess chlorine, 1,1,2,2-tetrachloropropane is obtained. Two chlorine molecules are added to \[{\text{C}} \equiv {\text{C}}\] triple bond.
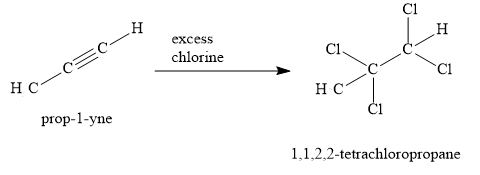
Thus, when 1,2-dichloropropane reacts with \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] and excess of chlorine, the product obtained is 1,1,2,2-tetrachloropropane.
Hence, the given statement is true. So, enter 1.
Note: Soda amide can also be used to abstract the most acidic proton of terminal alkynes. When a terminal alkyne loses an acidic proton, it forms an acetylide ion. This acetylide ion acts as a nucleophile and can attack carbonyl compounds to form carbon-carbon bonds.
Complete step by step answer:
You can classify halogen derivatives of alkanes into mono halogen derivatives, di halogen derivatives, tri halogen derivatives, tetra halogen derivatives and poly halogen derivatives. mono halogen derivatives, di halogen derivatives, tri halogen derivatives, tetra halogen derivatives and poly halogen derivatives of alkanes contain one, two, three, four and several halogen atoms. Halogen can be either fluorine or chlorine or bromine or iodine.
\[{\text{NaN}}{{\text{H}}_{\text{2}}}\] is soda amide. It is a strong base. It is used for the dehydrohalogenation of haloalkanes. During dehydrohalogenation, hydrogen halide molecules are eliminated and carbon-carbon multiple bonds are obtained. Mono halogen derivative of alkane loses one molecule of hydrogen halide and forms an alkene. Dihalogen derivative of alkane loses two molecules of hydrogen halide and forms an alkyne.
1,2-dichloropropane is the dichloro derivative of alkane. When 1,2-dichloropropane is treated with \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] , it loses two molecules of hydrogen chloride to form propyne (an alkyne).

When propyne is treated with excess chlorine, 1,1,2,2-tetrachloropropane is obtained. Two chlorine molecules are added to \[{\text{C}} \equiv {\text{C}}\] triple bond.

Thus, when 1,2-dichloropropane reacts with \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] and excess of chlorine, the product obtained is 1,1,2,2-tetrachloropropane.
Hence, the given statement is true. So, enter 1.
Note: Soda amide can also be used to abstract the most acidic proton of terminal alkynes. When a terminal alkyne loses an acidic proton, it forms an acetylide ion. This acetylide ion acts as a nucleophile and can attack carbonyl compounds to form carbon-carbon bonds.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




