
Statement: The amorphous solids are isotropic.
State whether the given statement is true or false.
(A) True
(B) False
Answer
580.8k+ views
Hint: Amorphous solids have regular shape. They are called Pseudo solids because there is no long range order in them. The value of physical properties would be the same along any direction.
Complete answer:
Amorphous means no form in Greek language.
The arrangement of constituent particles in such solids is irregular.
So they are called short range solid.
In this type of arrangement a regular pattern is observed over short distance only.
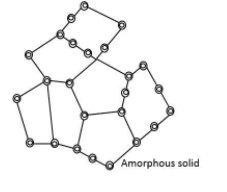
Since the arrangement of particles is different along different directions, the value of the physical properties is found to be the same along each direction. The property remains the same in all directions. This property is known as isotropy.
Therefore, the statement amorphous solids are isotropic in nature is true.
In the above diagram atoms are not orderly arranged.
The disarrangement is the same in any direction therefore physical properties along every direction is the same.
Therefore, from the above explanation the correct option is (A) True.
Note: Anisotropy is opposite property of isotropy.
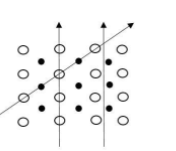
In crystalline solids particles are arranged in a regular manner.
In this diagram the arrangement is orderly. So when we go any direction, the arrangement will be different. Therefore, physical properties like electrical conductance are different in different directions.
Amorphous solids are useful material. Glass, Rubber and plastic are amorphous solids.
Complete answer:
Amorphous means no form in Greek language.
The arrangement of constituent particles in such solids is irregular.
So they are called short range solid.
In this type of arrangement a regular pattern is observed over short distance only.

Since the arrangement of particles is different along different directions, the value of the physical properties is found to be the same along each direction. The property remains the same in all directions. This property is known as isotropy.
Therefore, the statement amorphous solids are isotropic in nature is true.
In the above diagram atoms are not orderly arranged.
The disarrangement is the same in any direction therefore physical properties along every direction is the same.
Therefore, from the above explanation the correct option is (A) True.
Note: Anisotropy is opposite property of isotropy.

In crystalline solids particles are arranged in a regular manner.
In this diagram the arrangement is orderly. So when we go any direction, the arrangement will be different. Therefore, physical properties like electrical conductance are different in different directions.
Amorphous solids are useful material. Glass, Rubber and plastic are amorphous solids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




