
Statement I: ${{Buta - 1,3 - diene}}$ is less stable than ${{penta - 1,4 - diene}}$.
Statement II: ${{Buta - 1,3 - diene}}$ has a greater number of resonating structures and the delocalized electron cloud.
A.Statement- ${\rm I}$ is true, Statement -${\rm I}{\rm I}$ is true; Statement ${\rm I}{\rm I}$ is a correct explanation for Statement-${\rm I}$
B.Statement- ${\rm I}$ is true, Statement -${\rm I}{\rm I}$ is true; Statement ${\rm I}{\rm I}$ is not a correct explanation for Statement-${\rm I}$
C.Statement- ${\rm I}$ is true, Statement -${\rm I}{\rm I}$ is false
D.Statement- ${\rm I}$ is false, Statement -${\rm I}{\rm I}$ is true
Answer
579k+ views
Hint: The compound having lone pairs, double or triple bond have a maximum chance of having resonance. The compound having a large number of resonating structures is more stable. The conjugate double bond makes the compound more stable as seen in hyperconjugation.
Complete step by step answer:
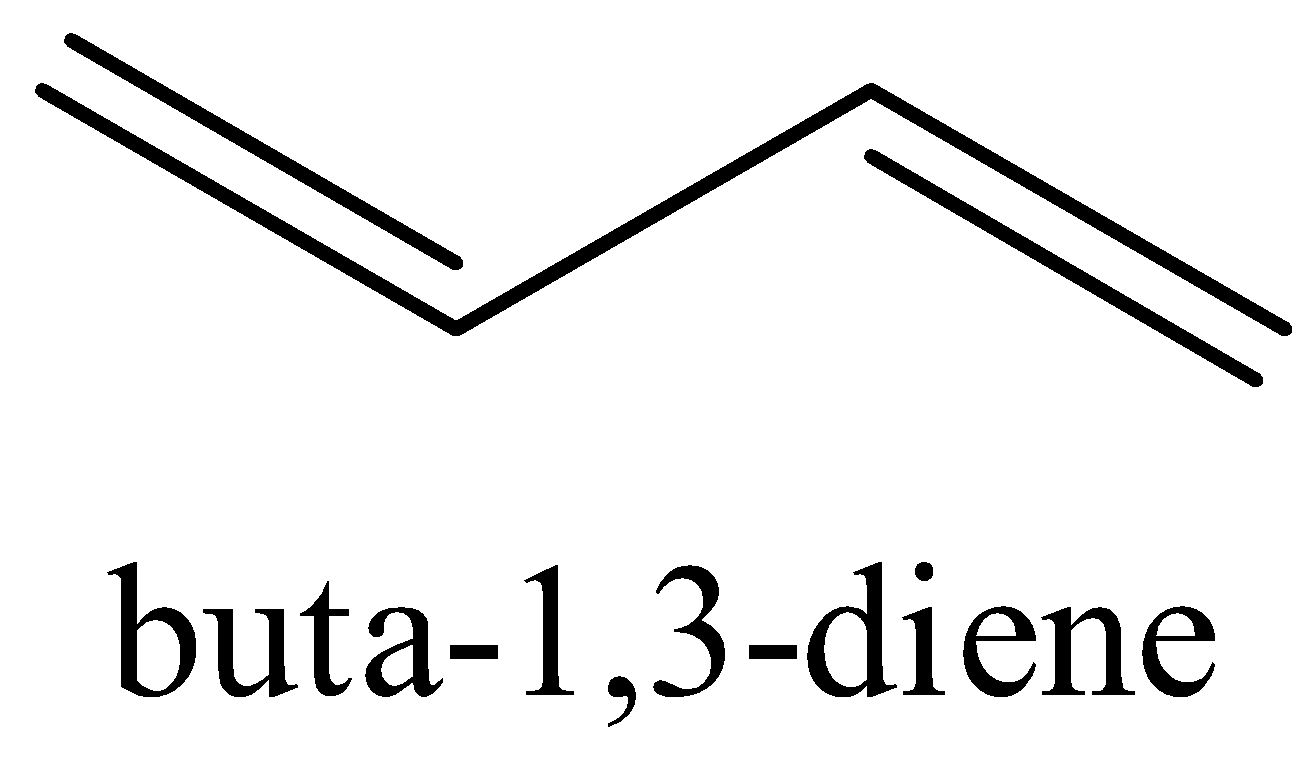
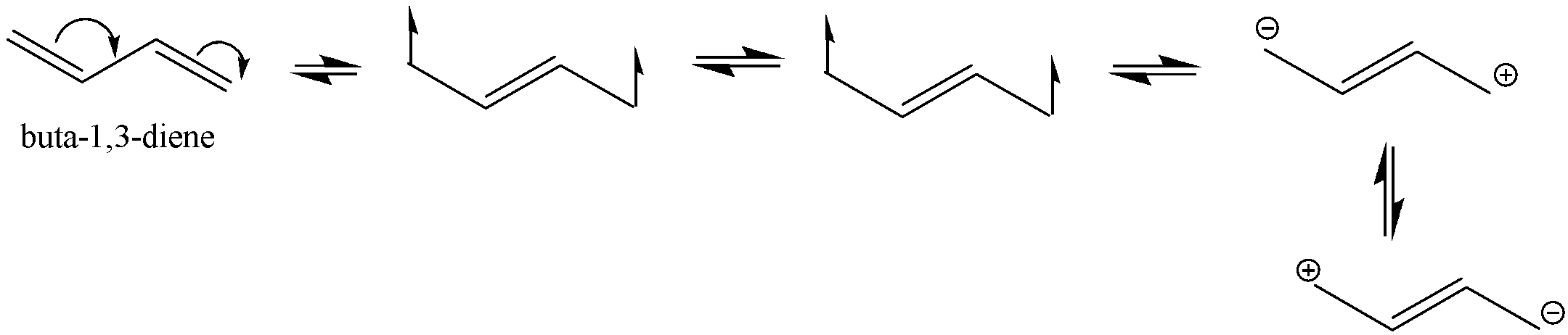
The structure ${{Buta - 1,3 - diene}}$ is shown below with its all resonating structures.
We can see that there are conjugated double bonds in ${{Buta - 1,3 - diene}}$ which means that the single bond and double bond alternates.
Thus, it leads to resonance structures. It enables the electrons to be delocalized to the whole system. We know that a molecule with more resonance structures is more stable.
The structure ${{penta - 1,4 - diene}}$ is shown below
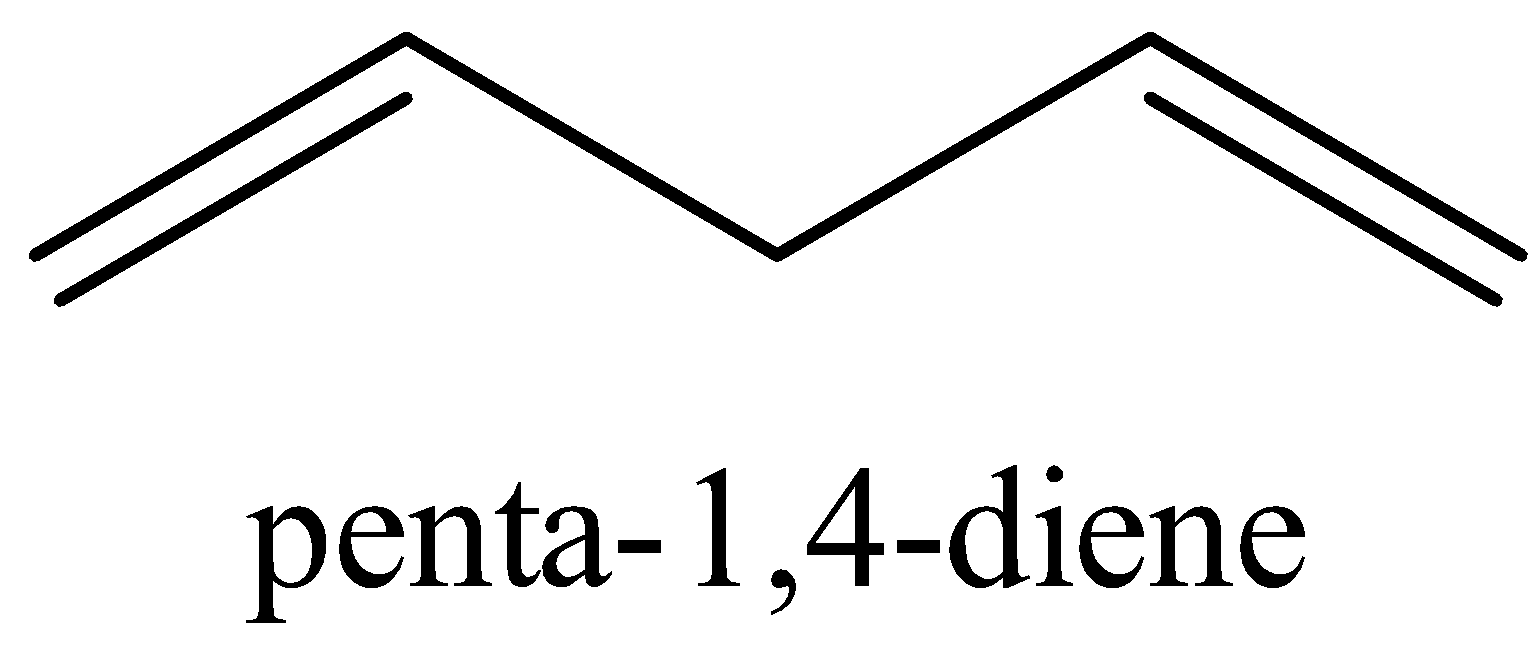
We could see that there are no conjugated double bonds in ${{penta - 1,4 - diene}}$.
Thus, it does not have resonance structures.
As we can see that ${{Buta - 1,3 - diene}}$ has 4 resonating structures and ${{penta - 1,4 - diene}}$ does not show resonance.
So we can conclude that ${{Buta - 1,3 - diene}}$ is more stable since it shows resonance. The more the resonating structures, the more is the stability.
Hence the statement ${\rm I}$ is false and the statement ${\rm I}{\rm I}$ is true.
Hence, the correct option is D.
Additional information: The stability of carbocation is as follows:- ${{tertiary > secondary > primary}}$. The compound having less energy is more stable. The compound with high energy is less stable.
Note:
To find the stability between two compounds the first focus should be to find the number of resonating structures shown by the compounds. If the compound is having more conjugate bonds then it is more stable. A Conjugate system is a system of bonds having p orbitals with delocalized electrons in a molecule.
Complete step by step answer:
The structure ${{Buta - 1,3 - diene}}$ is shown below with its all resonating structures.
We can see that there are conjugated double bonds in ${{Buta - 1,3 - diene}}$ which means that the single bond and double bond alternates.
Thus, it leads to resonance structures. It enables the electrons to be delocalized to the whole system. We know that a molecule with more resonance structures is more stable.


The structure ${{penta - 1,4 - diene}}$ is shown below

We could see that there are no conjugated double bonds in ${{penta - 1,4 - diene}}$.
Thus, it does not have resonance structures.
As we can see that ${{Buta - 1,3 - diene}}$ has 4 resonating structures and ${{penta - 1,4 - diene}}$ does not show resonance.
So we can conclude that ${{Buta - 1,3 - diene}}$ is more stable since it shows resonance. The more the resonating structures, the more is the stability.
Hence the statement ${\rm I}$ is false and the statement ${\rm I}{\rm I}$ is true.
Hence, the correct option is D.
Additional information: The stability of carbocation is as follows:- ${{tertiary > secondary > primary}}$. The compound having less energy is more stable. The compound with high energy is less stable.
Note:
To find the stability between two compounds the first focus should be to find the number of resonating structures shown by the compounds. If the compound is having more conjugate bonds then it is more stable. A Conjugate system is a system of bonds having p orbitals with delocalized electrons in a molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




