
Statement 1: The structure of $S{O_3}$ shown by using more than one structural formula
Statement 2: $S{O_3}$ is very unstable and resonates between these possible structures.
A.Both statement 1 and statement 2 are correct and statement 2 is correct explanation of statement 1
B.Both statement 1 and statement 2 are correct and statement 2 is not the correct explanation of statement 1
C.Statement 1 is correct but statement 2 is not correct.
D.Statement 1 is not correct but statement 2 is correct.
E.Both the statement 1 and statement 2 are not correct
Answer
579k+ views
Hint: We know that sulfur trioxide is present in many canonical/resonance forms. We will study the canonical forms of sulfur trioxide and accordingly arrive at the answer as sulfur trioxide exists in different forms due to the possible resonance possible.
From the resonance structures, we will judge the two statements present and arrive at the correct answer.
Complete step by step answer:
We know that for sulfur trioxide it exists in different canonical/resonance forms so we get different structures of sulfur trioxide and the same can be represented by using more than one structural formula. The more the number of resonating structures for a particular molecule, the more is the stability.
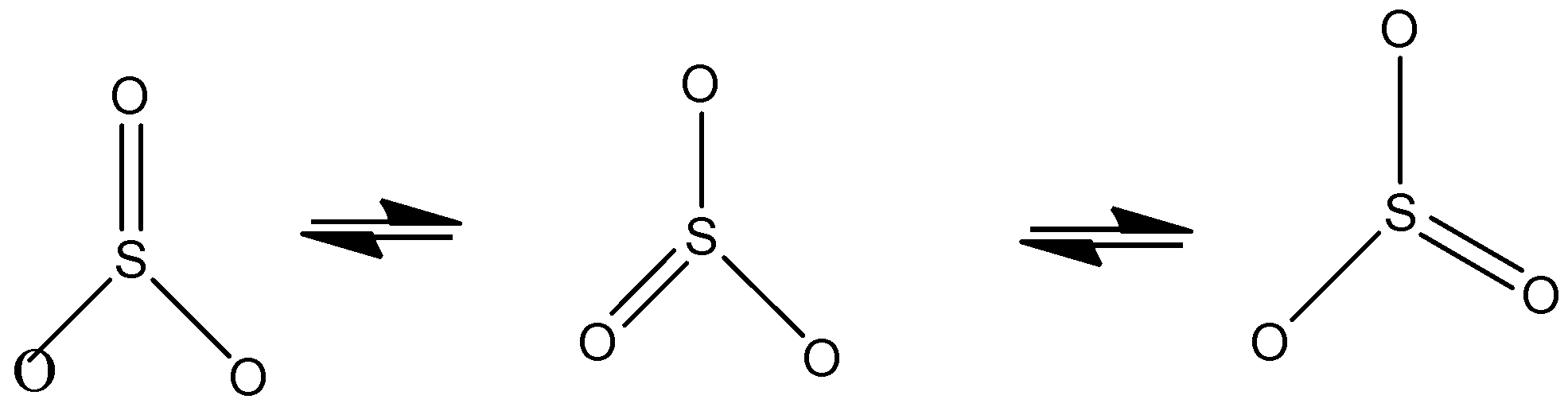
So statement 1 is correct
Also due to resonance each sulfur oxygen bond in sulfur trioxide molecule has a partial double bond character. It gains extra stability due to resonance.
So statement two is wrong.
Since statement one is correct and statement two is wrong.
Hence, the correct option is C.
Additional information: Gaseous sulfur trioxide is a trigonal planar molecule. Also it is an anhydride of sulfuric acid. It is a highly reactive substance and a strong oxidizing agent and also acts as a fire hazard. Sulfur trioxide behaves as the primary substance in an acid rain or we can say it is a pollutant in gaseous form.
Note:
Sulphur trioxide is indeed shown by three structures because here each bond is a hybrid of a single and a double bond. The extra stability present here is due to resonance as we know that resonance increases stability and also the same properties can be represented by different resonance structures.
From the resonance structures, we will judge the two statements present and arrive at the correct answer.
Complete step by step answer:
We know that for sulfur trioxide it exists in different canonical/resonance forms so we get different structures of sulfur trioxide and the same can be represented by using more than one structural formula. The more the number of resonating structures for a particular molecule, the more is the stability.

So statement 1 is correct
Also due to resonance each sulfur oxygen bond in sulfur trioxide molecule has a partial double bond character. It gains extra stability due to resonance.
So statement two is wrong.
Since statement one is correct and statement two is wrong.
Hence, the correct option is C.
Additional information: Gaseous sulfur trioxide is a trigonal planar molecule. Also it is an anhydride of sulfuric acid. It is a highly reactive substance and a strong oxidizing agent and also acts as a fire hazard. Sulfur trioxide behaves as the primary substance in an acid rain or we can say it is a pollutant in gaseous form.
Note:
Sulphur trioxide is indeed shown by three structures because here each bond is a hybrid of a single and a double bond. The extra stability present here is due to resonance as we know that resonance increases stability and also the same properties can be represented by different resonance structures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




