
State with suitable examples of Huckel’s rule of aromatic character.
Answer
588k+ views
Hint: Four structural criteria must be satisfied for a compound to be aromatic. The compound must be cyclic, conjugated, planar, and satisfies Huckel’s rule. Electrons in the p orbital are called $\pi $ electrons. Also we can confirm whether an atom is ${\text{s}}{{\text{p}}^2}$ hybridized by checking whether the atom has bonded to three atoms and it has no lone pairs.
Complete step by step answer:
Aromatic compounds have a distinctive odor. For a compound to be aromatic, it should be cyclic, planar with resonance bonds, not contain ${\text{s}}{{\text{p}}^3}$ hybridized carbon atom and satisfies Huckel’s rule.
When the compound has a ring of atoms, it is called a cyclic compound. It is said to be planar if all the atoms are in the same plane.
Huckel’s rule states that the aromatic compounds contain $\left( {4{\text{n}} + 2} \right)\pi $ electrons, where ${\text{n}}$ is any integer.
We know that benzene is an aromatic compound. It has three double bonds and each double bond has two electrons each. So a total of six $\pi $ electrons are there in benzene.
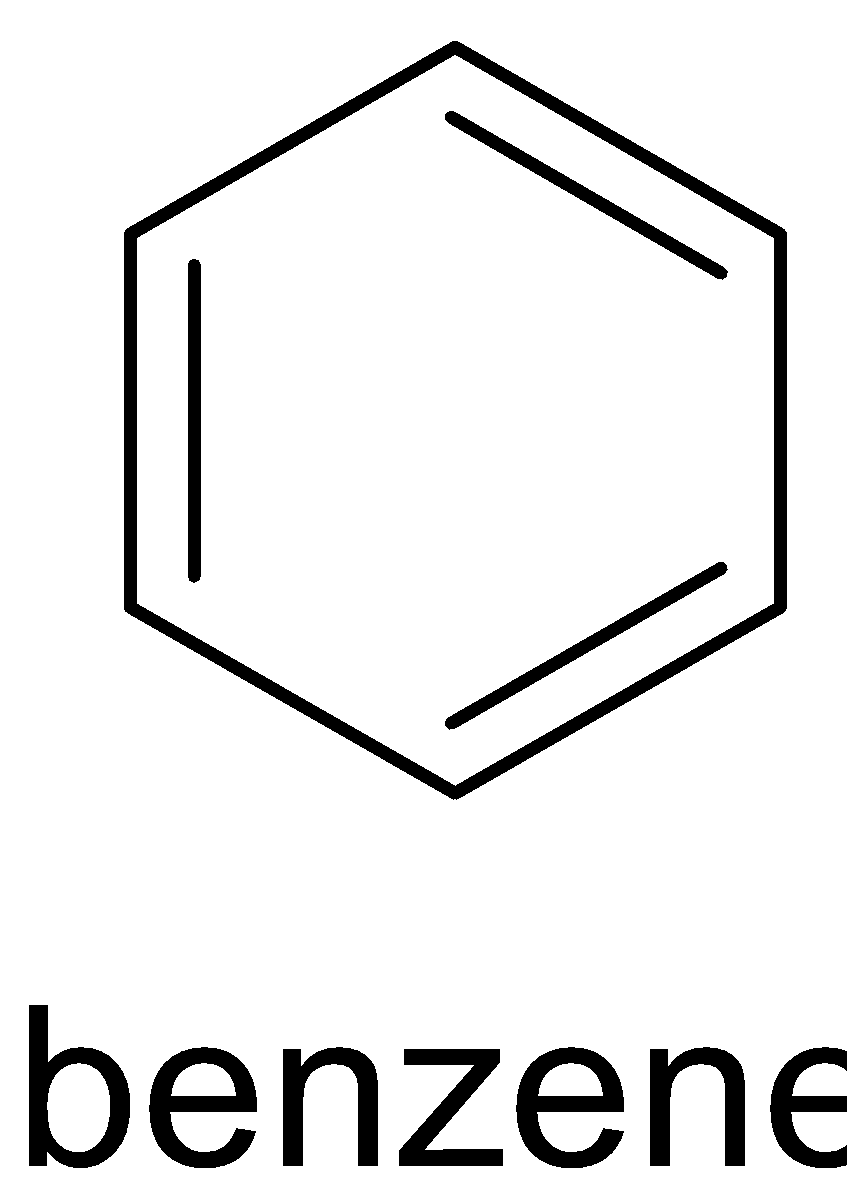
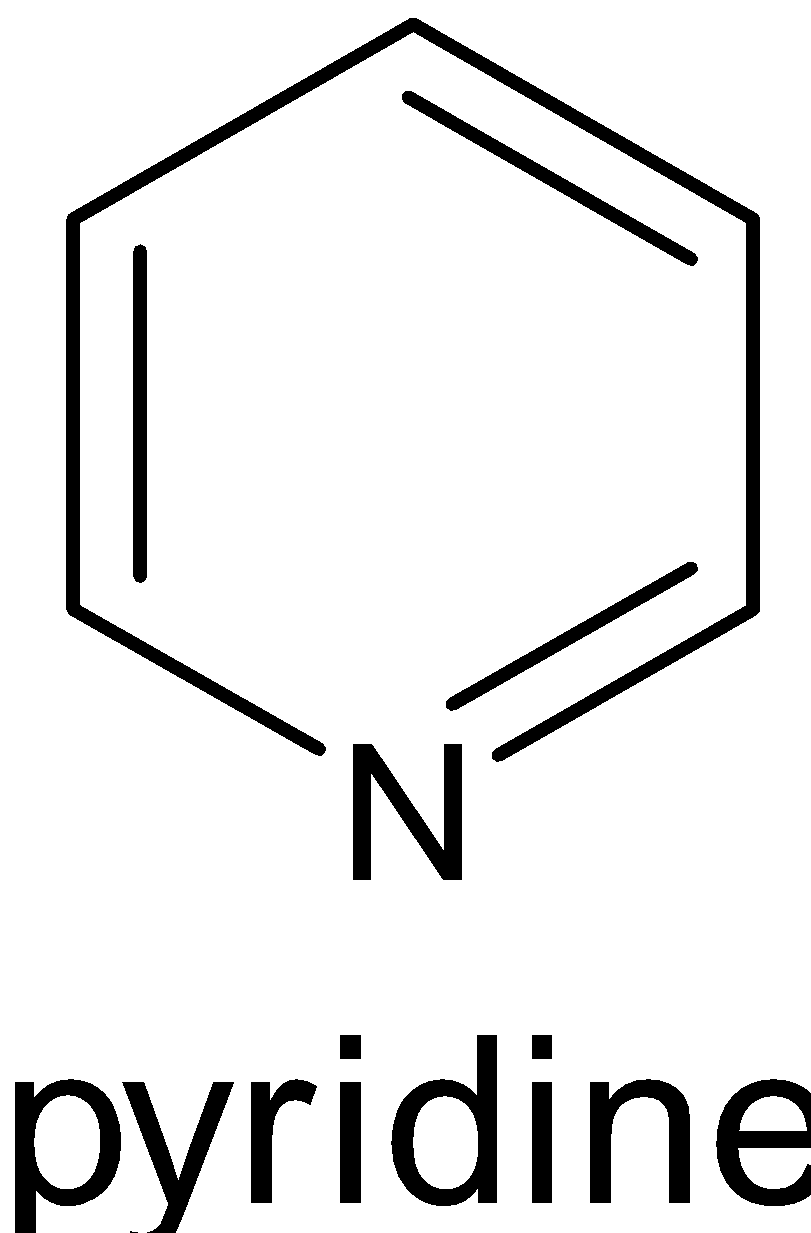
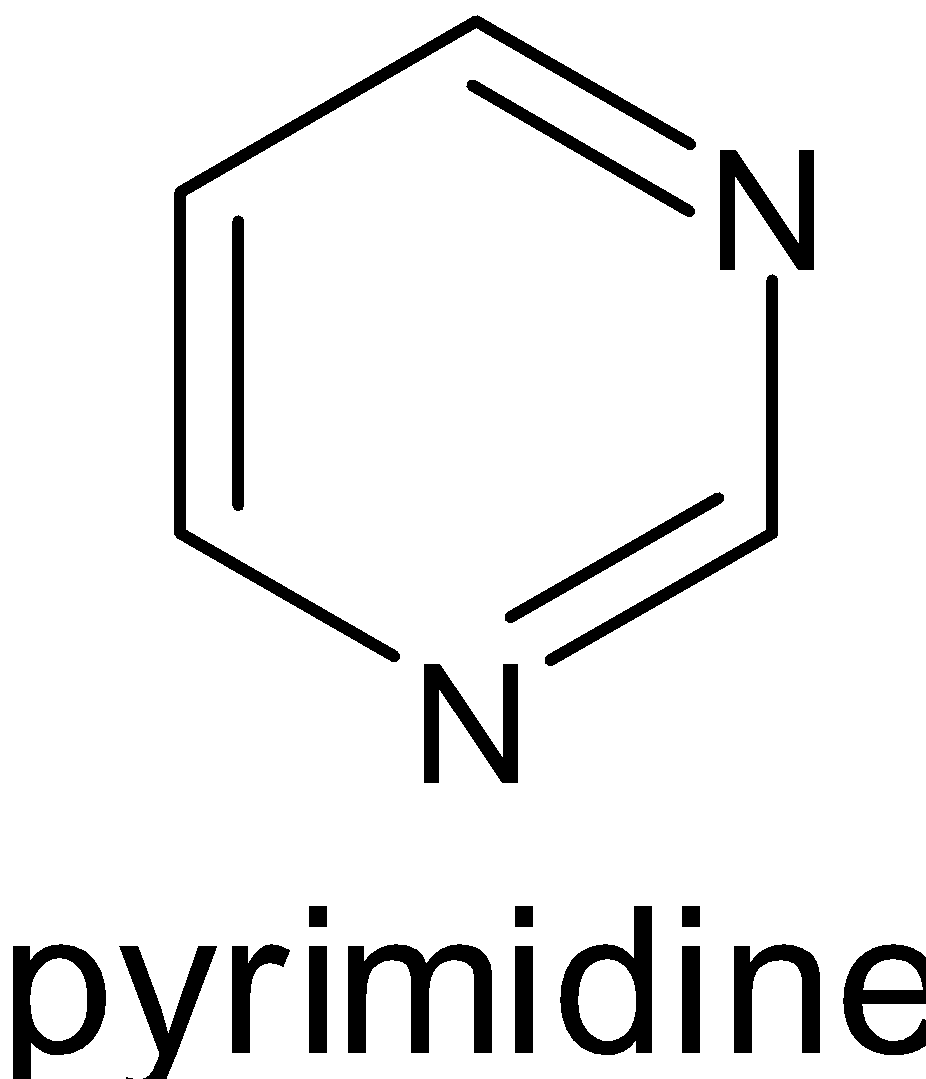
Three of them have six $\pi $ electrons, where ${\text{n}} = 1$
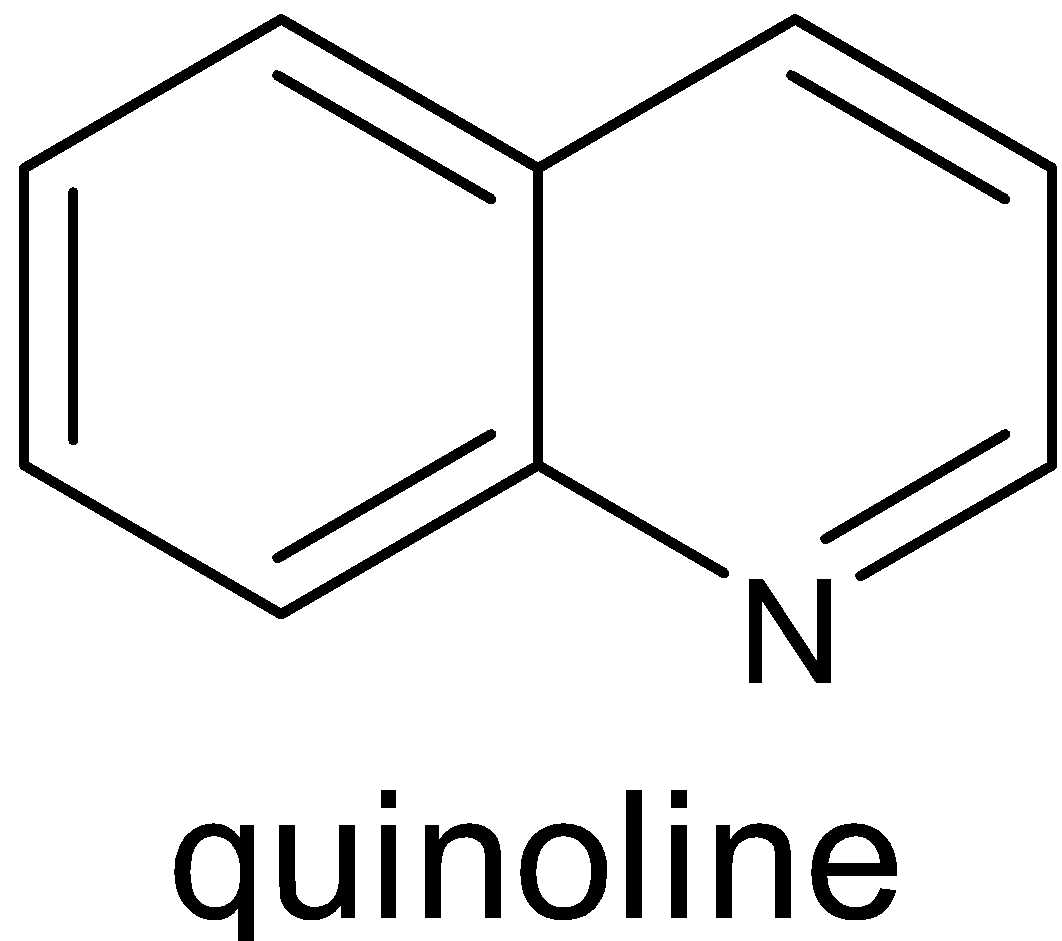
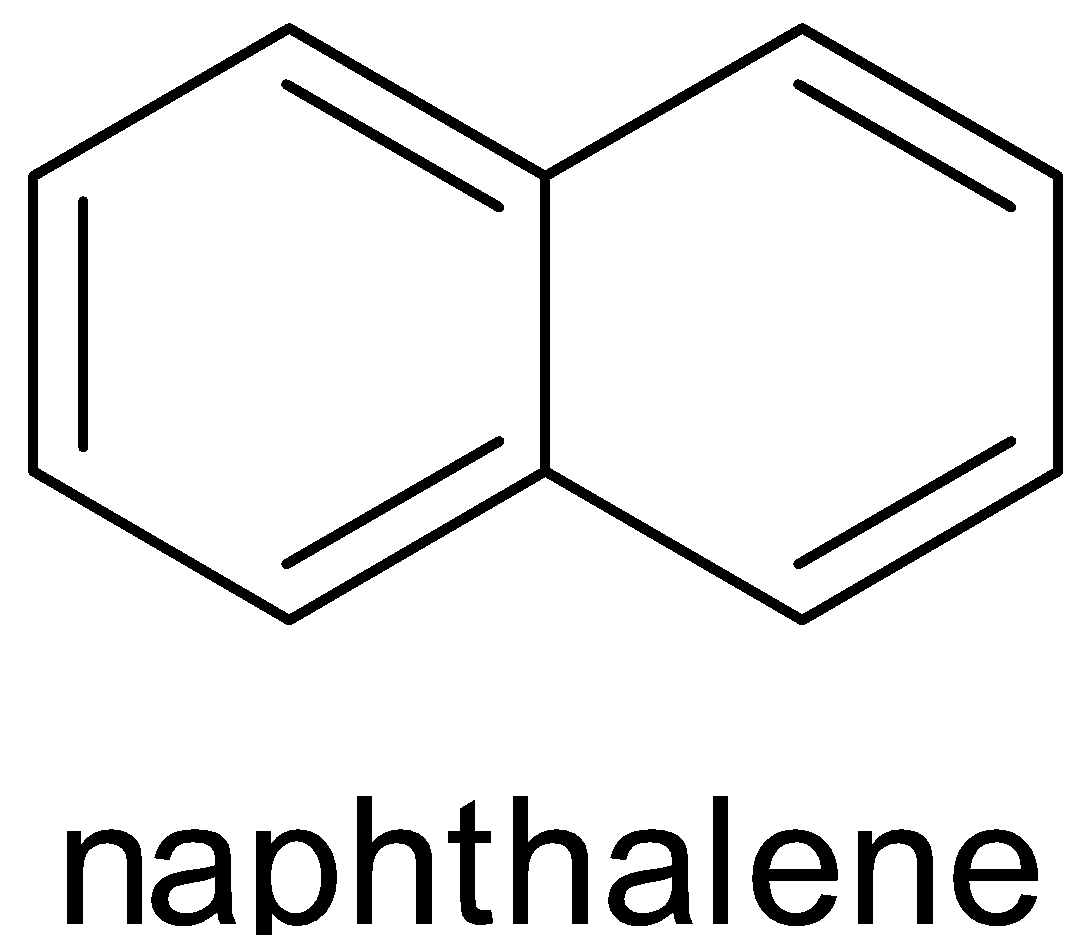
Both of them have ten $\pi $ electrons.
$ 4{\text{n}} + 2 = 10 \\
4{\text{n}} = 8 \\
{\text{n}} = 2 \\ $
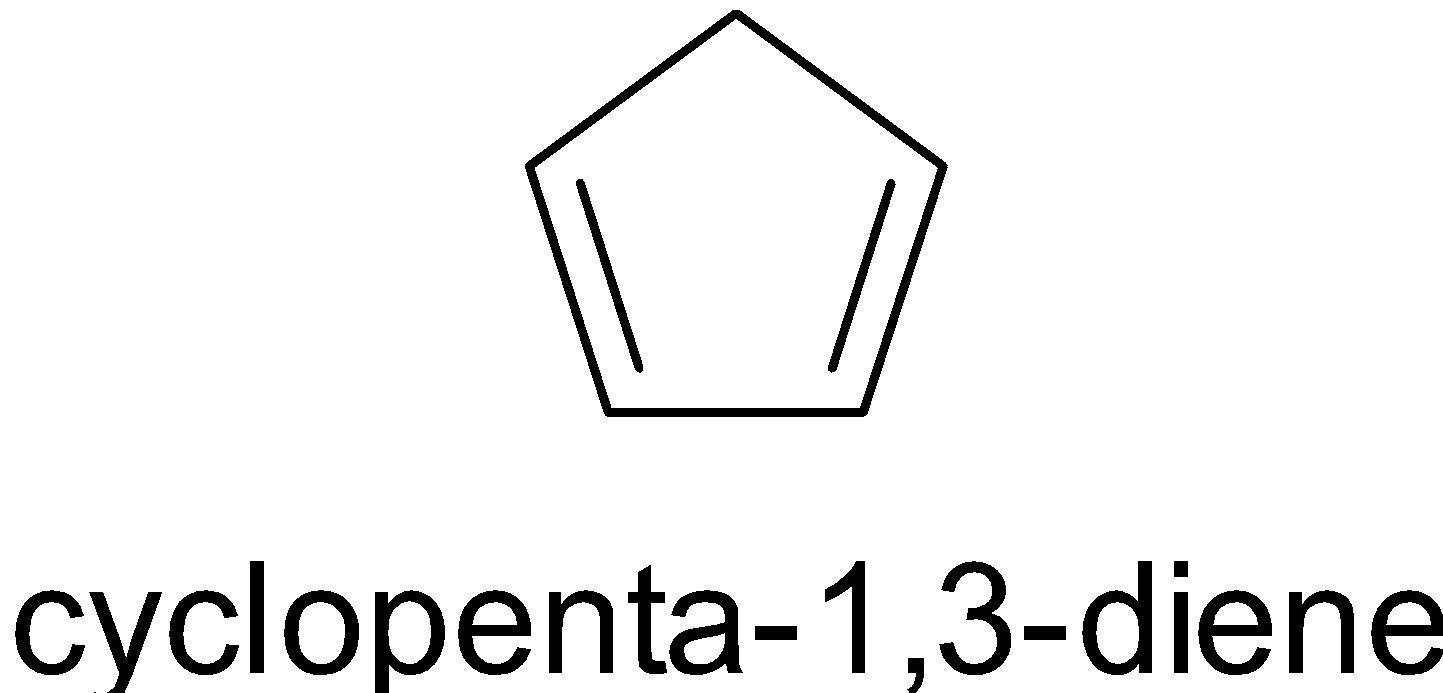 is not aromatic since the carbon on top is ${\text{s}}{{\text{p}}^3}$ hybridized.
is not aromatic since the carbon on top is ${\text{s}}{{\text{p}}^3}$ hybridized.
Additional information:
$\pi $ electrons are on the p orbitals. We can tell the compound is conjugated, if all of its molecules are ${\text{s}}{{\text{p}}^2}$ hybridized. This indicates that it is fully conjugated.
Note:
Based on Huckel’s molecular orbital theory, when all the bonding orbitals are filled with electrons, it is said to be stable. In aromatic compounds, two electrons are filled in the lower energy orbital and four electrons are filled in the following energy level.
Complete step by step answer:
Aromatic compounds have a distinctive odor. For a compound to be aromatic, it should be cyclic, planar with resonance bonds, not contain ${\text{s}}{{\text{p}}^3}$ hybridized carbon atom and satisfies Huckel’s rule.
When the compound has a ring of atoms, it is called a cyclic compound. It is said to be planar if all the atoms are in the same plane.
Huckel’s rule states that the aromatic compounds contain $\left( {4{\text{n}} + 2} \right)\pi $ electrons, where ${\text{n}}$ is any integer.
We know that benzene is an aromatic compound. It has three double bonds and each double bond has two electrons each. So a total of six $\pi $ electrons are there in benzene.



Three of them have six $\pi $ electrons, where ${\text{n}} = 1$


Both of them have ten $\pi $ electrons.
$ 4{\text{n}} + 2 = 10 \\
4{\text{n}} = 8 \\
{\text{n}} = 2 \\ $

Additional information:
$\pi $ electrons are on the p orbitals. We can tell the compound is conjugated, if all of its molecules are ${\text{s}}{{\text{p}}^2}$ hybridized. This indicates that it is fully conjugated.
Note:
Based on Huckel’s molecular orbital theory, when all the bonding orbitals are filled with electrons, it is said to be stable. In aromatic compounds, two electrons are filled in the lower energy orbital and four electrons are filled in the following energy level.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




