
State Avogadro’s Law.
Answer
594k+ views
Hint: A relation between number of moles and volume at constant temperature and pressure is given by V = K n, where ‘n’ is the number of moles of the gas and V is the volume of the gas.
Complete answer:
Avogadro’s law (volume-amount relationship)
This law was given by an Italian Scientist Amedeo Avogadro in the year 1811. He put forward a relation between the volume and number of molecules at constant temperature and pressure. It was experimentally found that the volume of a gas is directly proportional to the number of moles keeping temperature and pressure constant.I.e. V ∝ n (T and P constant)
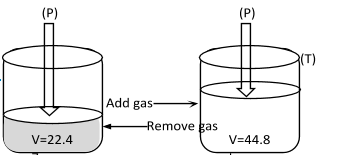
Figure showing V ∝ n at T, P constant.
Where ‘n’ is the number of moles of the gas.
Or V = Kn
Or $\dfrac{V}{n} = K$
Where K is proportionality constant, now if two gases with volumes $V_1$ and $V_2$ and moles $n_1$ and $n_2$ at constant temperature and pressure are taken then \[\dfrac{{{V_1}}}{{{n_1}}} = \dfrac{{{V_2}}}{{{n_2}}} = K\]
It is evident from the above relation with if the volume of two gases is equal then number of moles is also equal
I.e. if $V_1$ = $V_2$
Then $n_1$ is also equal to $n_2$ ($n_1$ = $n_2$)
In this way Avogadro’s law states that the equal volume of all gases under the same condition of temperature and pressure contain equal number of moles or molecules
Note:As the ideal gas equation expresses the quantitative relation between the four variables that describe the state of a gas, therefore it is called the equation of state for gases.
Complete answer:
Avogadro’s law (volume-amount relationship)
This law was given by an Italian Scientist Amedeo Avogadro in the year 1811. He put forward a relation between the volume and number of molecules at constant temperature and pressure. It was experimentally found that the volume of a gas is directly proportional to the number of moles keeping temperature and pressure constant.I.e. V ∝ n (T and P constant)

Figure showing V ∝ n at T, P constant.
Where ‘n’ is the number of moles of the gas.
Or V = Kn
Or $\dfrac{V}{n} = K$
Where K is proportionality constant, now if two gases with volumes $V_1$ and $V_2$ and moles $n_1$ and $n_2$ at constant temperature and pressure are taken then \[\dfrac{{{V_1}}}{{{n_1}}} = \dfrac{{{V_2}}}{{{n_2}}} = K\]
It is evident from the above relation with if the volume of two gases is equal then number of moles is also equal
I.e. if $V_1$ = $V_2$
Then $n_1$ is also equal to $n_2$ ($n_1$ = $n_2$)
In this way Avogadro’s law states that the equal volume of all gases under the same condition of temperature and pressure contain equal number of moles or molecules
Note:As the ideal gas equation expresses the quantitative relation between the four variables that describe the state of a gas, therefore it is called the equation of state for gases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




