
What is the space lattice of diamond?
A) Fcc
B) Bcc with two atoms per unit cell
C) Bcc with 4 atoms per unit cell
D) Simple cubic
Answer
574.5k+ views
Hint: Space lattice also known as Bravais lattice. It is a regular array of points (commonly atoms/ions) in a three-dimensional object. Each point in space lattice refers to an atom or group of atoms. In this question we have to determine the space lattice of diamonds. The ionic structure of diamond consists of a two-atomic basis. One of the two atoms is sitting on the lattice point and the other one is shifted by 14 along each axis.
Complete answer:
Diamond is a crystal structure with a face centred cubic space lattice and two identical atoms in the basis. One atom sits on the lattice point whereas the other one is shifted by 14 along each axis. This forms a tetrahedral structure where each atom is surrounded by four equal-distanced neighbours. Each atom forms bonds with four nearest neighbours. The enclosed angles between two angles are 109.47°. The distance between the two atoms equals one-quarter of the body diagonal of the cube. The diamond lattice contains also 4 Bravais points per unit cell but contains 8 atoms per unit cell tetrahedrally bonded atoms in each primitive cell.
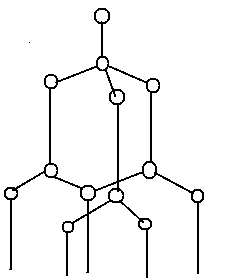
Therefore, option A is the correct answer.
Additional Information: Face-centred cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centres of each face of the unit cell. It has unit cell vectors $a = b = c$ and interaxial angles $\alpha = \beta = \gamma = {90^o}$. Whereas Body-centred cubic lattice (bcc or cubic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional point at the centre of the cell. It has unit cell vectors $a = b = c$ and interaxial angles $\alpha = \beta = \gamma = {90^o}$.
Note: We have to remember that the face-centered cubic (fcc) has a coordination number of 12 and contains 4 atoms per unit cell. But a body-centered cubic (bcc) has a coordination number of 8 and contains 2 atoms per unit cell.
Complete answer:
Diamond is a crystal structure with a face centred cubic space lattice and two identical atoms in the basis. One atom sits on the lattice point whereas the other one is shifted by 14 along each axis. This forms a tetrahedral structure where each atom is surrounded by four equal-distanced neighbours. Each atom forms bonds with four nearest neighbours. The enclosed angles between two angles are 109.47°. The distance between the two atoms equals one-quarter of the body diagonal of the cube. The diamond lattice contains also 4 Bravais points per unit cell but contains 8 atoms per unit cell tetrahedrally bonded atoms in each primitive cell.

Therefore, option A is the correct answer.
Additional Information: Face-centred cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centres of each face of the unit cell. It has unit cell vectors $a = b = c$ and interaxial angles $\alpha = \beta = \gamma = {90^o}$. Whereas Body-centred cubic lattice (bcc or cubic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional point at the centre of the cell. It has unit cell vectors $a = b = c$ and interaxial angles $\alpha = \beta = \gamma = {90^o}$.
Note: We have to remember that the face-centered cubic (fcc) has a coordination number of 12 and contains 4 atoms per unit cell. But a body-centered cubic (bcc) has a coordination number of 8 and contains 2 atoms per unit cell.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




