
Some meta-directing substituents in aromatic substitution are given. Which one is most deactivating?
(A) $-C\equiv N$
(B) $-S{{O}_{3}}H$
(C) $-COOH$
(D) $-N{{O}_{2}}$
Answer
579k+ views
Hint: Before determining the strength of the groups mentioned in options, identify the meta position with respect to a directing group. Now test the strength of each of these groups for their ability to withdraw the electrons from the aromatic system. Based on this you can determine the most deactivating group.
Complete step by step solution:
Electronic factors are the factor that influence various organic reactions and rearrangements. Electronic effects are significantly observed in organic aromatic compounds.
Electronic factors are:
- Inductive effect
- Resonance
- Mesomeric effect
- Electromeric effect
- Hyperconjugation.
We will now identify at which position of the aromatic ring , the following effects are observed.
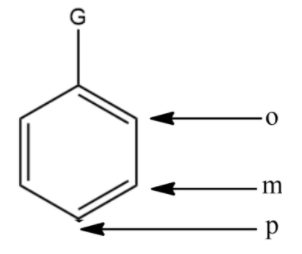
In the above compound,
- o stands for ortho position
- m stands for meta position
- p stands for para position
In the above system, the hydroxyl group acts as the directing group Now that we have identified the meta position, we will determine the strength of the groups given in the options above.
(A) $-C\equiv N$: The cyanide group withdraws electrons from the aromatic system by resonance. It is a strong deactivating group.
(B) $-S{{O}_{3}}H$: The sulfonyl group withdraws electrons from the aromatic system by resonance. It is a strong deactivating group as well.
(C) $-COOH$: The carboxylic group withdraws electrons from the aromatic system by resonance. However, it is considered to be a moderately deactivating group.
(D) $-N{{O}_{2}}$ : The nitro group withdraws electrons from the aromatic system through resonance as well as inductive effect. It is considered to be the strongest deactivating group.
Based on the above explanation, we can conclude that the most deactivating meta substituting group is $-N{{O}_{2}}$.
Therefore, the correct answer is option (D).
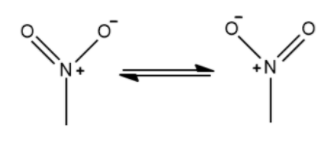
Note: It is important to know that the nitro group is considered to be the strongest deactivating group due to the equal resonating structure formed which reduces the electron charge density to a greater extent. The diagram is given below for better understanding:
Complete step by step solution:
Electronic factors are the factor that influence various organic reactions and rearrangements. Electronic effects are significantly observed in organic aromatic compounds.
Electronic factors are:
- Inductive effect
- Resonance
- Mesomeric effect
- Electromeric effect
- Hyperconjugation.
We will now identify at which position of the aromatic ring , the following effects are observed.

In the above compound,
- o stands for ortho position
- m stands for meta position
- p stands for para position
In the above system, the hydroxyl group acts as the directing group Now that we have identified the meta position, we will determine the strength of the groups given in the options above.
(A) $-C\equiv N$: The cyanide group withdraws electrons from the aromatic system by resonance. It is a strong deactivating group.
(B) $-S{{O}_{3}}H$: The sulfonyl group withdraws electrons from the aromatic system by resonance. It is a strong deactivating group as well.
(C) $-COOH$: The carboxylic group withdraws electrons from the aromatic system by resonance. However, it is considered to be a moderately deactivating group.
(D) $-N{{O}_{2}}$ : The nitro group withdraws electrons from the aromatic system through resonance as well as inductive effect. It is considered to be the strongest deactivating group.
Based on the above explanation, we can conclude that the most deactivating meta substituting group is $-N{{O}_{2}}$.
Therefore, the correct answer is option (D).
Note: It is important to know that the nitro group is considered to be the strongest deactivating group due to the equal resonating structure formed which reduces the electron charge density to a greater extent. The diagram is given below for better understanding:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




