
Sodium alkylbenzene sulfonate is used as:
(a)- Soap
(b)- Fertilizer
(c)- Detergent
(d)- Pesticide
Answer
558.9k+ views
Hint: Sodium alkylbenzene sulfonate is a class of surfactants in which the alkylbenzene sulfonates are the anionic part and the sodium is the cationic part so, basically it is the anionic surfactant. Since it is surfactant it is used for cleaning purposes. It can react with both soft water and hard water also.
Complete step by step answer:
Sodium alkylbenzene sulfonate is a class of surfactants in which the alkylbenzene sulfonates are the anionic part and the sodium is the cationic part so, basically, it is the anionic surfactant. The structure of sodium alkylbenzene sulfonate is given below:
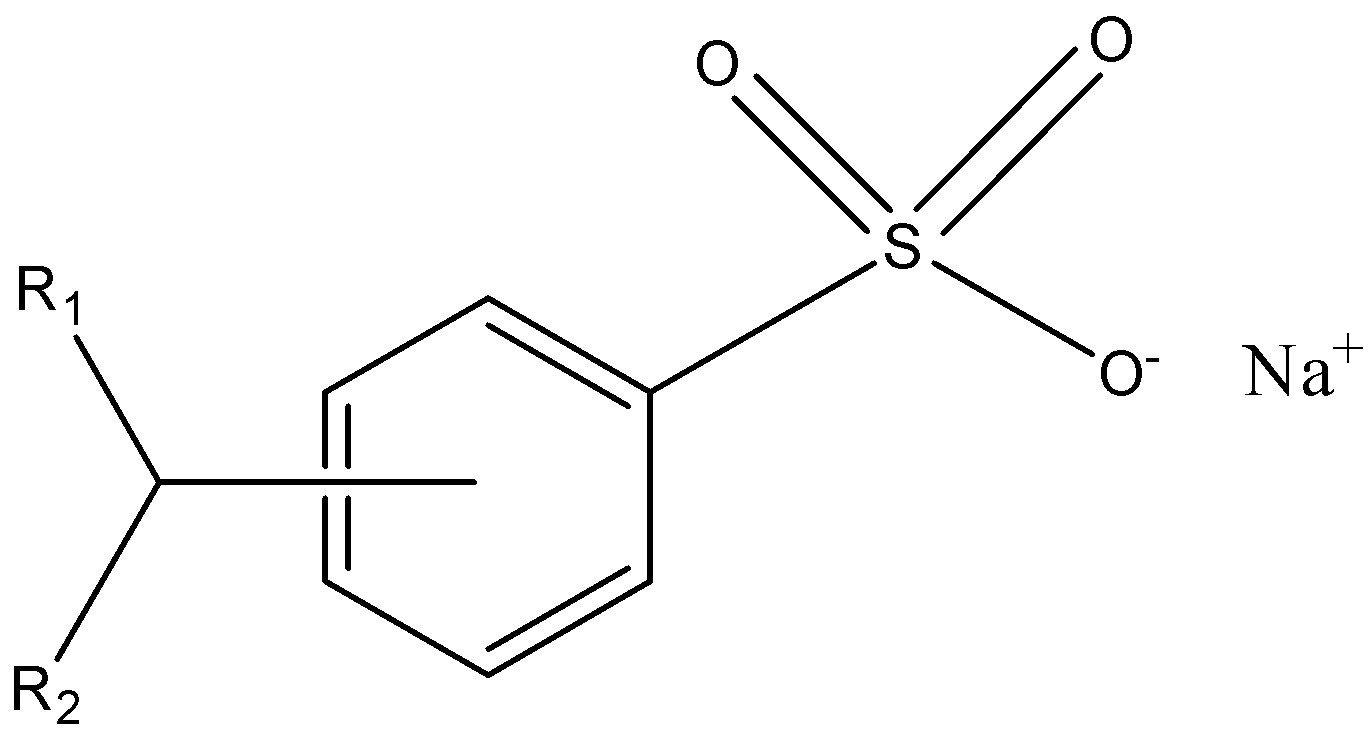
- In this molecule, the sulfonate group is the head and it is the hydrophilic part, which means that it is water liking, and alkylbenzene is the tail part and it is the hydrophobic part, which means that it is water-hating.
- It is used for cleaning and it is soluble in soft as well as hard water, therefore it is a detergent. It is a synthetic detergent and is one of the oldest ones. It is also used in shampoos, toothpaste, dishwashing liquid.
- As it is a detergent and they are produced chemically, they are less biodegradable because of the presence of sulfonate group which is not easily detached by the micro-organism in the sewage. The correct option is option “C” .
Note: Detergents can react with both soft water and hard water, due to which it does not form the scum with water. Some other examples of detergents are sodium lauryl sulfate and deoxycholic acid, etc.
Complete step by step answer:
Sodium alkylbenzene sulfonate is a class of surfactants in which the alkylbenzene sulfonates are the anionic part and the sodium is the cationic part so, basically, it is the anionic surfactant. The structure of sodium alkylbenzene sulfonate is given below:

- In this molecule, the sulfonate group is the head and it is the hydrophilic part, which means that it is water liking, and alkylbenzene is the tail part and it is the hydrophobic part, which means that it is water-hating.
- It is used for cleaning and it is soluble in soft as well as hard water, therefore it is a detergent. It is a synthetic detergent and is one of the oldest ones. It is also used in shampoos, toothpaste, dishwashing liquid.
- As it is a detergent and they are produced chemically, they are less biodegradable because of the presence of sulfonate group which is not easily detached by the micro-organism in the sewage. The correct option is option “C” .
Note: Detergents can react with both soft water and hard water, due to which it does not form the scum with water. Some other examples of detergents are sodium lauryl sulfate and deoxycholic acid, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




