
What is soap $?$ Describe its preparation.
Answer
541.5k+ views
Hint :Soap, a surfactant is a form of lipid which is a mixture of sodium salts of various naturally occurring higher fatty acids like palmitic acid $ ({C_{15}}{H_{31}}COOH) $ i.e. fatty acids having long carbon chain. Soap can be made from the base hydrolysis of a fat or an oil, the process known as saponification.
Complete Step By Step Answer:
Soap is a form of lipid and is a mixture of sodium salts of various naturally occurring higher fatty acids. A fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Soaps generally contain higher fatty acids having long carbon chains like palmitic acid $ ({C_{15}}{H_{31}}COOH) $ .
Soap is made from the base hydrolysis of a fat or an oil and this process of hydrolysis is called saponification.
When fats and oils are triesters of glycerol and three fatty acids are hydrolysed, esters are hydrolyzed to their alcohol and carboxylic acid components in the presence of bases like $ NaOH $ or $ KOH $ . Since a base is used for hydrolysis, the fatty acids produced are deprotonated and are present as the corresponding carboxylate salts. Glycerol is obtained as the side product in the reaction. The equation corresponding to saponification is:
$ Triesters\,of\,glycerol\,and\,three\,fatty\,acids\, + \,3\,NaOH\, \to \,Glycerol\, + \,Soap $ .
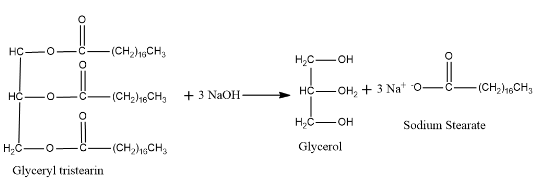
Example: When glycerol tristearin, a fat is hydrolysed we get sodium stearate, a soap and glycerol as the side product.
Note :
Detergents are similar to soaps in that they have a charged head group and a long nonpolar tail group, but they are not prepared from natural fats or oils. Soaps are less effective in hard water which contains a significant concentration of $ C{a^{ + 2}} $ and $ M{g^{ + 2}} $ ions as these ions form precipitates with soap molecules. Soaps are effective in soft water where formation of precipitate is less. While detergents are useful because they do not form precipitates with $ C{a^{ + 2}} $ and $ M{g^{ + 2}} $ ions, which means that they work in both soft and hard water.
Complete Step By Step Answer:
Soap is a form of lipid and is a mixture of sodium salts of various naturally occurring higher fatty acids. A fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Soaps generally contain higher fatty acids having long carbon chains like palmitic acid $ ({C_{15}}{H_{31}}COOH) $ .
Soap is made from the base hydrolysis of a fat or an oil and this process of hydrolysis is called saponification.
When fats and oils are triesters of glycerol and three fatty acids are hydrolysed, esters are hydrolyzed to their alcohol and carboxylic acid components in the presence of bases like $ NaOH $ or $ KOH $ . Since a base is used for hydrolysis, the fatty acids produced are deprotonated and are present as the corresponding carboxylate salts. Glycerol is obtained as the side product in the reaction. The equation corresponding to saponification is:
$ Triesters\,of\,glycerol\,and\,three\,fatty\,acids\, + \,3\,NaOH\, \to \,Glycerol\, + \,Soap $ .
Example: When glycerol tristearin, a fat is hydrolysed we get sodium stearate, a soap and glycerol as the side product.

Note :
Detergents are similar to soaps in that they have a charged head group and a long nonpolar tail group, but they are not prepared from natural fats or oils. Soaps are less effective in hard water which contains a significant concentration of $ C{a^{ + 2}} $ and $ M{g^{ + 2}} $ ions as these ions form precipitates with soap molecules. Soaps are effective in soft water where formation of precipitate is less. While detergents are useful because they do not form precipitates with $ C{a^{ + 2}} $ and $ M{g^{ + 2}} $ ions, which means that they work in both soft and hard water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




