
$S{{O}_{3}}$ is an electrophile in the substitution of benzene.
(A) True
(B) False
Answer
590.1k+ views
Hint: Sulphonation of benzene is a reversible reaction. A mixture of sulphur trioxide and sulphuric acid is used as a reacting mixture. The sulphur in sulphur trioxide delocalised the double bond in benzene to form a phenyl intermediate leading to formation of benzene sulphonic acid.
Complete step by step answer:
Benzene is reactive towards electrophilic substitution reactions compared to addition reactions as it loses its aromaticity during addition reaction as it leads to formation of highly unstable intermediate.
As benzene contains delocalized electrons across the carbon atoms present in the ring, it attracts incoming electrophiles and forms a stable intermediate in substitution reactions. In general, the electrophilic substitution reaction of benzene consists of a three-step process which involves:
- Generation of the electrophile.
- Intermediate carbocation formation.
- Removal of a proton from carbocation intermediate.
Sulfonation of benzene is a process of heating benzene with hot fuming sulphuric acid or oleum (${{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ + S}{{\text{O}}_{\text{3}}}$) to produce benzenesulfonic acid. This is a reversible reaction.
Due to higher electronegativity, oxygen present in sulphuric acid attracts the electron cloud towards itself, leading to formation of electrophile. This electrophile attacks the benzene ring, leading to the formation of benzenesulfonic acid. The reaction is given below:
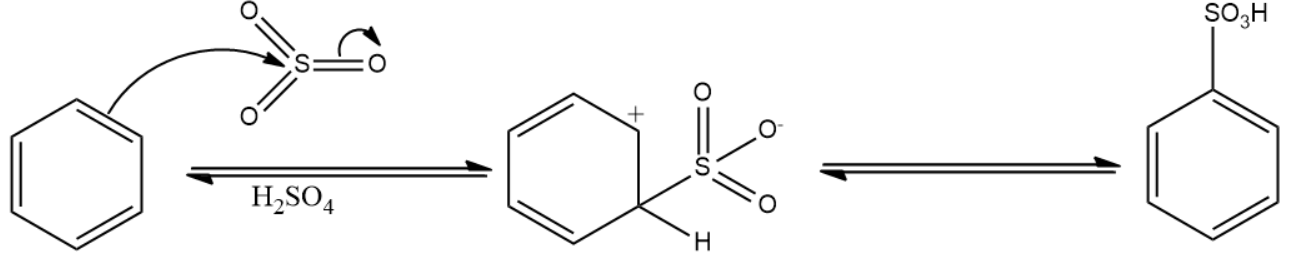
Note: Mixture of conc.$HN{{O}_{3}}$ and conc.${{H}_{2}}S{{O}_{4}}$ is commonly referred to as the nitrating mixture. Nitrating mixture is used to attach a nitro group to the benzene ring. The reaction is given below:
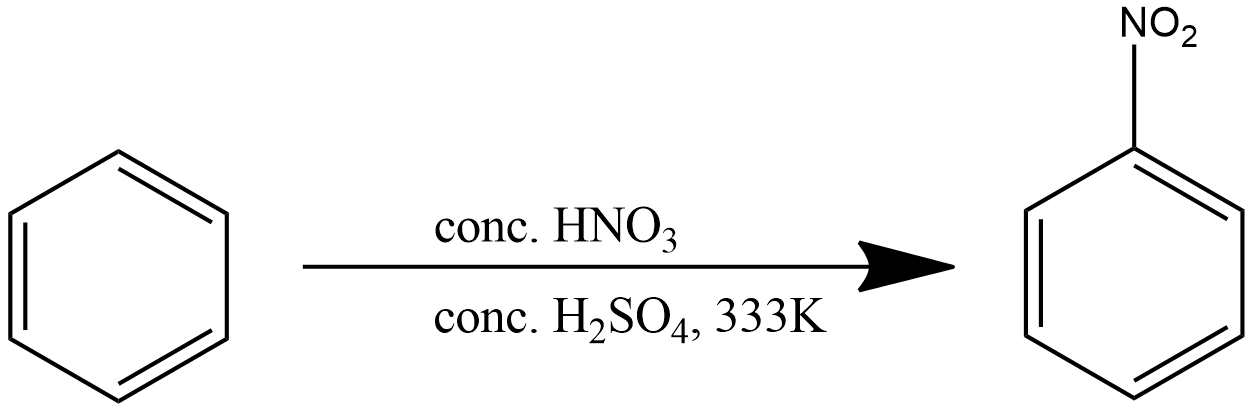
When a nitrating mixture is added to a derivative of benzene, the nitro group is attached to either the para or ortho position of the dominant functional group.
However, nitration of benzene or its derivatives is not part of electrophilic aromatic substitution, as the nitrating mixture does not increase the nucleophilicity of the benzene ring which is a characteristic feature of EAS electrophiles.
Complete step by step answer:
Benzene is reactive towards electrophilic substitution reactions compared to addition reactions as it loses its aromaticity during addition reaction as it leads to formation of highly unstable intermediate.
As benzene contains delocalized electrons across the carbon atoms present in the ring, it attracts incoming electrophiles and forms a stable intermediate in substitution reactions. In general, the electrophilic substitution reaction of benzene consists of a three-step process which involves:
- Generation of the electrophile.
- Intermediate carbocation formation.
- Removal of a proton from carbocation intermediate.
Sulfonation of benzene is a process of heating benzene with hot fuming sulphuric acid or oleum (${{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{ + S}{{\text{O}}_{\text{3}}}$) to produce benzenesulfonic acid. This is a reversible reaction.
Due to higher electronegativity, oxygen present in sulphuric acid attracts the electron cloud towards itself, leading to formation of electrophile. This electrophile attacks the benzene ring, leading to the formation of benzenesulfonic acid. The reaction is given below:

Note: Mixture of conc.$HN{{O}_{3}}$ and conc.${{H}_{2}}S{{O}_{4}}$ is commonly referred to as the nitrating mixture. Nitrating mixture is used to attach a nitro group to the benzene ring. The reaction is given below:
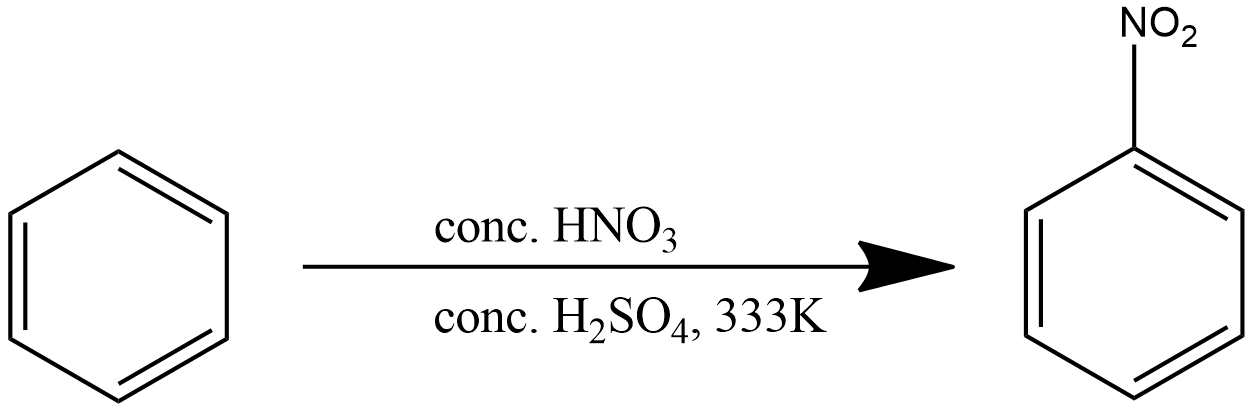
When a nitrating mixture is added to a derivative of benzene, the nitro group is attached to either the para or ortho position of the dominant functional group.
However, nitration of benzene or its derivatives is not part of electrophilic aromatic substitution, as the nitrating mixture does not increase the nucleophilicity of the benzene ring which is a characteristic feature of EAS electrophiles.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




