
Silicon’s are synthetic polymers containing repeated ${{R}_{2}}SiO$ units.
(A) True
(B) False
Answer
585k+ views
Hint: Polymers are macromolecules built up by linking together a large number of small molecules. The repeating units in a polymer are linked through strong covalent bonds. Small molecules combined to form polymer molecules are called monomers. Carbon and silicon are from group 14 in the periodic table that silicon also forms polymers like carbon because of its catenation property.
Complete step by step solution:
Generally, polymers are classified into many types based on their physical and chemical properties.
The classification of polymers is:
-Based on source
-Based on polymerization
-Based on functionality
-Based on intermolecular forces
-Based on monomers
-Based on the source:
Polymers are classified into two types based on availability of source or abundance in nature. Those are natural polymers and synthetic polymers.
Natural polymers: those polymers are available in nature such as plants and animals.
Examples: proteins, cellulose, starch, some resins, and natural rubber.
Synthetic polymers: These varieties of synthetic polymers as plastics, synthetic fibers, and synthetic rubbers, silicon’s are examples of man-made polymers prepared in daily life as well as in industry or laboratories.
Silicones:
These polymers are known as synthetic organ silicon polymers, which contain repeated ${{R}_{2}}SiO$ units by Si-O-Si linkages.
Silicones compounds have a general formula ${{({{R}_{2}}SiO)}_{n}}$, where R = alkyl or aryl group.
Polymerization of Silicones:
Step-1: when silicon reacts with methyl chloride in presence of copper catalysts at 570K, various types of methyl-substituted chlorosilanes $C{{H}_{3}}SiC{{l}_{3}},{{(C{{H}_{3}})}_{2}}SiC{{l}_{2}},{{(C{{H}_{3}})}_{3}}SiCl,{{(C{{H}_{3}})}_{4}}Si$ are formed.
Step-2: hydrolysis ${{(C{{H}_{3}})}_{2}}SiC{{l}_{2}}$ is followed by polymerization yields chain polymer.
${{(C{{H}_{3}})}_{2}}SiC{{l}_{2}}+{{H}_{2}}O\to {{(C{{H}_{3}})}_{2}}Si{{(OH)}_{2}}+2HCl$
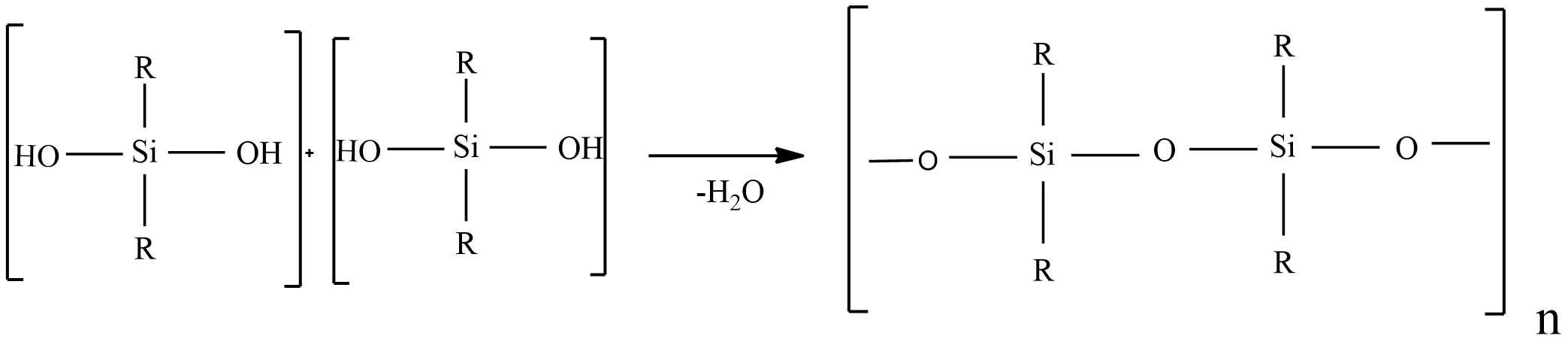
So, the given statement Silicon’s are synthetic polymers containing repeated \[{{R}_{2}}SiO\] units is true.
The correct option is A.
Note: Silicones are made up of short-chain molecules that are oily liquid, medium chains behave as viscous liquids, greases and very long chains behave as elastomers and resins. These are chemically inert and easily attacked by organic reagents. These are heat resistant and have a high dielectric strength.
Complete step by step solution:
Generally, polymers are classified into many types based on their physical and chemical properties.
The classification of polymers is:
-Based on source
-Based on polymerization
-Based on functionality
-Based on intermolecular forces
-Based on monomers
-Based on the source:
Polymers are classified into two types based on availability of source or abundance in nature. Those are natural polymers and synthetic polymers.
Natural polymers: those polymers are available in nature such as plants and animals.
Examples: proteins, cellulose, starch, some resins, and natural rubber.
Synthetic polymers: These varieties of synthetic polymers as plastics, synthetic fibers, and synthetic rubbers, silicon’s are examples of man-made polymers prepared in daily life as well as in industry or laboratories.
Silicones:
These polymers are known as synthetic organ silicon polymers, which contain repeated ${{R}_{2}}SiO$ units by Si-O-Si linkages.
Silicones compounds have a general formula ${{({{R}_{2}}SiO)}_{n}}$, where R = alkyl or aryl group.
Polymerization of Silicones:
Step-1: when silicon reacts with methyl chloride in presence of copper catalysts at 570K, various types of methyl-substituted chlorosilanes $C{{H}_{3}}SiC{{l}_{3}},{{(C{{H}_{3}})}_{2}}SiC{{l}_{2}},{{(C{{H}_{3}})}_{3}}SiCl,{{(C{{H}_{3}})}_{4}}Si$ are formed.
Step-2: hydrolysis ${{(C{{H}_{3}})}_{2}}SiC{{l}_{2}}$ is followed by polymerization yields chain polymer.
${{(C{{H}_{3}})}_{2}}SiC{{l}_{2}}+{{H}_{2}}O\to {{(C{{H}_{3}})}_{2}}Si{{(OH)}_{2}}+2HCl$

So, the given statement Silicon’s are synthetic polymers containing repeated \[{{R}_{2}}SiO\] units is true.
The correct option is A.
Note: Silicones are made up of short-chain molecules that are oily liquid, medium chains behave as viscous liquids, greases and very long chains behave as elastomers and resins. These are chemically inert and easily attacked by organic reagents. These are heat resistant and have a high dielectric strength.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




