
How many sigma and pi bonds do sp, $s{p^2}$, $s{p^3}$, $s{p^3}d$, $s{p^3}{d^2}$ have?
Answer
556.5k+ views
Hint: A single bond formed between the two atoms containing one sigma bond, a double bond formed between the two atoms containing one sigma and one pi bond. A triple bond formed between the two atoms containing one sigma and two pi bonds.
Complete step by step answer:
The property by which the atomic orbitals fuse with each other to form new hybridized orbitals is known as hybridization.
From the hybridization of the central atom, one can know the number of sigma bonds around the central atom.
In acetylene molecules, the sp hybridization is seen which contains two sigma bonds around one carbon atom and two pi bonds around one carbon atom.
The structure of acetylene is shown below.
$H - C \equiv C - H$
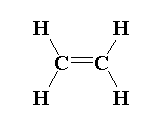
In ethene molecules, $s{p^2}$ hybridization is seen where three sigma bonds are present around one carbon and one pi bond is present around one carbon atom.
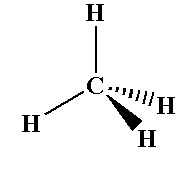
The methane molecule shows $s{p^3}$ hybridization where four sigma bonds are present around one carbon and no pi bond present.
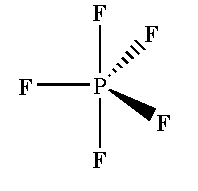
In phosphorus pentafluoride $P{F_5}$ molecule $s{p^3}d$ hybridization is seen where five sigma bonds are present around one phosphorus atom and no pi bonds are present.
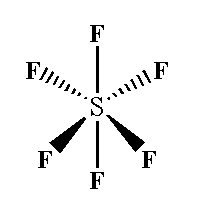
In sulfur hexafluoride $S{F_6}$, $s{p^3}{d^2}$ hybridization is seen where six sigma bonds are present around the sulfur atom and no pi bonds are present.
Note:
The number of orbitals taking part in hybridization is the number of sigma bonds made around the central atom. In $s{p^3}$, $s{p^3}d$ and $s{p^3}{d^2}$ no pi bond is present as it contains only a single covalent bond.
Complete step by step answer:
The property by which the atomic orbitals fuse with each other to form new hybridized orbitals is known as hybridization.
From the hybridization of the central atom, one can know the number of sigma bonds around the central atom.
In acetylene molecules, the sp hybridization is seen which contains two sigma bonds around one carbon atom and two pi bonds around one carbon atom.
The structure of acetylene is shown below.
$H - C \equiv C - H$
In ethene molecules, $s{p^2}$ hybridization is seen where three sigma bonds are present around one carbon and one pi bond is present around one carbon atom.

The methane molecule shows $s{p^3}$ hybridization where four sigma bonds are present around one carbon and no pi bond present.

In phosphorus pentafluoride $P{F_5}$ molecule $s{p^3}d$ hybridization is seen where five sigma bonds are present around one phosphorus atom and no pi bonds are present.

In sulfur hexafluoride $S{F_6}$, $s{p^3}{d^2}$ hybridization is seen where six sigma bonds are present around the sulfur atom and no pi bonds are present.

Note:
The number of orbitals taking part in hybridization is the number of sigma bonds made around the central atom. In $s{p^3}$, $s{p^3}d$ and $s{p^3}{d^2}$ no pi bond is present as it contains only a single covalent bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




