
How many Sigma and Pi Bonds are there in \[HCONHC{H_3}\] ?
Answer
507.3k+ views
Hint: We know that a sigma bond is formed by the head-on overlap of two orbitals whereas a pi bond is formed when the orbitals overlap laterally. A single bond contains a single sigma bond, a double bond contains one sigma bond and one pi bond, a triple bond contains one sigma bond and two pi bonds.
Complete answer:
For Solving these kinds of questions always draw the complete structure of the molecule given. This will help us to easily count the number of single, double and triple bonds present in the molecule.
The structure of \[HCONHC{H_3}\] is given as follows:
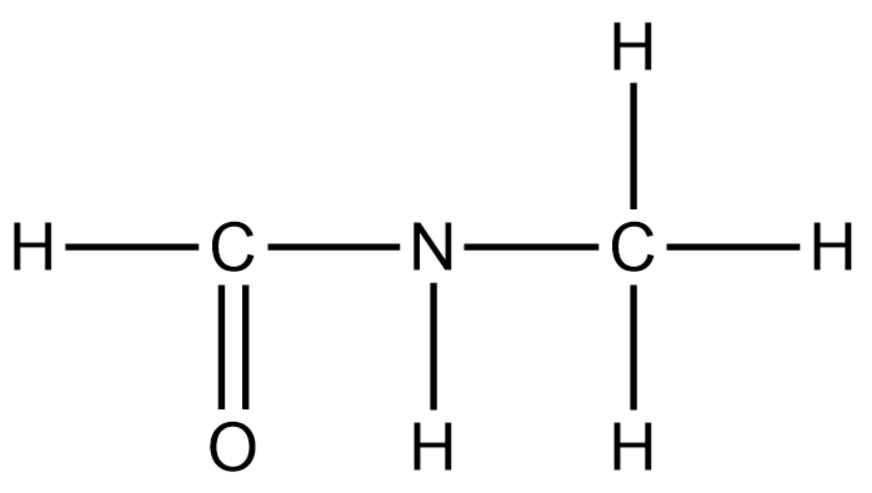
From the above figure we can easily say that there are 4 \[C - H\] single bonds, 1 \[N - H\] single bond, 2 \[C - N\] single bond and there is 1 \[C = O\] double bond.
Since each single bond contains one sigma ( \[\sigma \] ) bond and each double bond contains one sigma and one pi (\[\pi \] ) bond, we can say that there are 8 sigma bonds and 1 pi bond.
4 \[C - H\] single bonds \[ \Rightarrow \] 4 \[\sigma \]
1 \[N - H\] single bond \[ \Rightarrow \] 1 \[\sigma \]
2 \[C - N\] single bonds \[ \Rightarrow \] 2 \[\sigma \]
1 \[C = O\] double bond \[ \Rightarrow \] 1 \[\sigma \] and 1 \[\pi \]
Total: 8 \[\sigma \] and 1 \[\pi \] bond
Note:
Sigma bond is much stronger than the pi bond. This is because in sigma bonds the electron density is concentrated to a much larger extent between the two nuclei (Head-on overlap). In Pi bond this is not the case. There is electron density above and below the plane which makes electron density less concentrated and hence it is weaker than sigma.
Complete answer:
For Solving these kinds of questions always draw the complete structure of the molecule given. This will help us to easily count the number of single, double and triple bonds present in the molecule.
The structure of \[HCONHC{H_3}\] is given as follows:

From the above figure we can easily say that there are 4 \[C - H\] single bonds, 1 \[N - H\] single bond, 2 \[C - N\] single bond and there is 1 \[C = O\] double bond.
Since each single bond contains one sigma ( \[\sigma \] ) bond and each double bond contains one sigma and one pi (\[\pi \] ) bond, we can say that there are 8 sigma bonds and 1 pi bond.
4 \[C - H\] single bonds \[ \Rightarrow \] 4 \[\sigma \]
1 \[N - H\] single bond \[ \Rightarrow \] 1 \[\sigma \]
2 \[C - N\] single bonds \[ \Rightarrow \] 2 \[\sigma \]
1 \[C = O\] double bond \[ \Rightarrow \] 1 \[\sigma \] and 1 \[\pi \]
Total: 8 \[\sigma \] and 1 \[\pi \] bond
Note:
Sigma bond is much stronger than the pi bond. This is because in sigma bonds the electron density is concentrated to a much larger extent between the two nuclei (Head-on overlap). In Pi bond this is not the case. There is electron density above and below the plane which makes electron density less concentrated and hence it is weaker than sigma.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




