
Shape of P-orbital is:
A. Spherical
B. dumb-Bell
C. Double dumb bell
D. None of these
Answer
567.3k+ views
Hint:To determine the shape of p-orbitals we should know the structure of the p-orbital and the number of lobes present in the p-orbital. P- subshell has three orbits. Three orbital shells are denoted as ${{\text{P}}_{\text{X}}}$,${{\text{P}}_{\text{Y}}}$, and ${{\text{P}}_{\text{Z}}}$.
Complete step-by-step solution:The number of orbitals in a subshell is determined by the following formula:
${\text{2l}}\,{\text{ + }}\,{\text{1}}$
Where,
${\text{l}}\,$ is the azimuthal quantum number.
The l value of p-orbital is $1$.
So,
${\text{2}} \times {\text{1}}\,{\text{ + }}\,{\text{1}}$
$3$
The P-subshell has three, ${{\text{P}}_{\text{X}}}$,${{\text{P}}_{\text{Y}}}$and ${{\text{P}}_{\text{Z}}}$orbitals. All these orbitals are degenerate. All orbitals of the p-subshell lie on the axis. So, the electron density of each orbital also lies on the axis. Each p-orbital has two lobes.
The positions of p-orbitals on the axis is represented as follows:
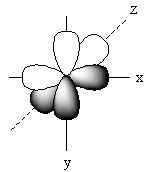
The positions of these three p-orbitals on the axis separately is represented as follows:
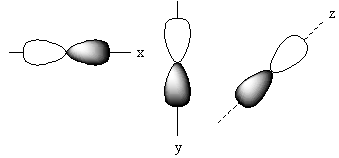
Name of each p-orbital depends upon the axis. The p-orbital which lies on the x-axis is known as ${{\text{P}}_{\text{x}}}$ orbital. The p-orbital which lies on the y-axis is known as ${{\text{P}}_{\text{Y}}}$ orbital. The p-orbital which lies on the z-axis is known as ${{\text{P}}_{\text{Z}}}$ orbital.
Each p-orbital has two lobes that are arranged like a dumb bell so, the shape of p–orbital is dumb bell.
So, the shape of P-orbital is dumb-Bell.
Therefore, option (B) dumb-Bell, is correct.
Note:The s-subshell has only one s-orbital. S-orbital has one lobe of spherical shape so, the shape of s-orbital is spherical. D-subshell has five d-orbitals. Out of five d-orbitals, two lie on the axis and three lie in between the axis. ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$and ${{\text{d}}_{{{\text{X}}^2} - {{\text{Y}}^{\text{2}}}}}$orbitals which lies on the axis and the ${{\text{d}}_{XZ}}$,${{\text{d}}_{YZ}}$, and ${{\text{d}}_{XY}}$ lies in between the axis. Except ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$all d-orbital has four lobes so, the shape of d-orbital is double dumb bell.
Complete step-by-step solution:The number of orbitals in a subshell is determined by the following formula:
${\text{2l}}\,{\text{ + }}\,{\text{1}}$
Where,
${\text{l}}\,$ is the azimuthal quantum number.
The l value of p-orbital is $1$.
So,
${\text{2}} \times {\text{1}}\,{\text{ + }}\,{\text{1}}$
$3$
The P-subshell has three, ${{\text{P}}_{\text{X}}}$,${{\text{P}}_{\text{Y}}}$and ${{\text{P}}_{\text{Z}}}$orbitals. All these orbitals are degenerate. All orbitals of the p-subshell lie on the axis. So, the electron density of each orbital also lies on the axis. Each p-orbital has two lobes.
The positions of p-orbitals on the axis is represented as follows:

The positions of these three p-orbitals on the axis separately is represented as follows:

Name of each p-orbital depends upon the axis. The p-orbital which lies on the x-axis is known as ${{\text{P}}_{\text{x}}}$ orbital. The p-orbital which lies on the y-axis is known as ${{\text{P}}_{\text{Y}}}$ orbital. The p-orbital which lies on the z-axis is known as ${{\text{P}}_{\text{Z}}}$ orbital.
Each p-orbital has two lobes that are arranged like a dumb bell so, the shape of p–orbital is dumb bell.
So, the shape of P-orbital is dumb-Bell.
Therefore, option (B) dumb-Bell, is correct.
Note:The s-subshell has only one s-orbital. S-orbital has one lobe of spherical shape so, the shape of s-orbital is spherical. D-subshell has five d-orbitals. Out of five d-orbitals, two lie on the axis and three lie in between the axis. ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$and ${{\text{d}}_{{{\text{X}}^2} - {{\text{Y}}^{\text{2}}}}}$orbitals which lies on the axis and the ${{\text{d}}_{XZ}}$,${{\text{d}}_{YZ}}$, and ${{\text{d}}_{XY}}$ lies in between the axis. Except ${{\text{d}}_{{{\text{z}}^{\text{2}}}}}$all d-orbital has four lobes so, the shape of d-orbital is double dumb bell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




